

Dot Blots of Alpha-synuclein antibodies
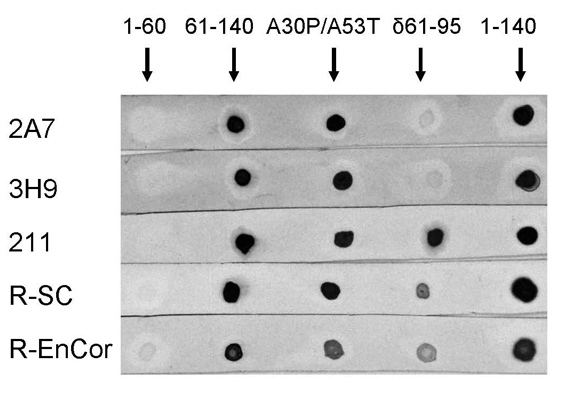
Figure: Various trancated and mutant construct were applied to PVDF membranes in about of 400ng of each protein. These were the first 60 amino acids of human alpha-synuclein (1-60), amino acids 61 to 140 (61-140), full length but incorporating the A30P and A53T mutations seen associated with familial forms of Parkinson's disease (A30P/A53T), full length but with the central NAC region of amino acids 61-95 missing (d61-95) and finally full length alpha synuclein. The strips of PVDF were probed with MCA-2A7, MCA-3H9, the Santa Cruz monoclonal antibody 211, the Santa Cruz rabbit polyclonal (R-SC) and EnCor's rabbit polyclonal (R-EnCor). The epitopes for MCA-2A7 and MCA-3H9 are clearly in the NAC region from 61-95, while the 211 antibody epitope is within the C-terminal region from 95-140 (in fact the epitope has been shown to be aminoacids 120-125). Both rabbit antibodies bind constructs including amino acids 61-140, but do not appear to bind amino acids 1-60, suggesting that this region is not immunogenic.