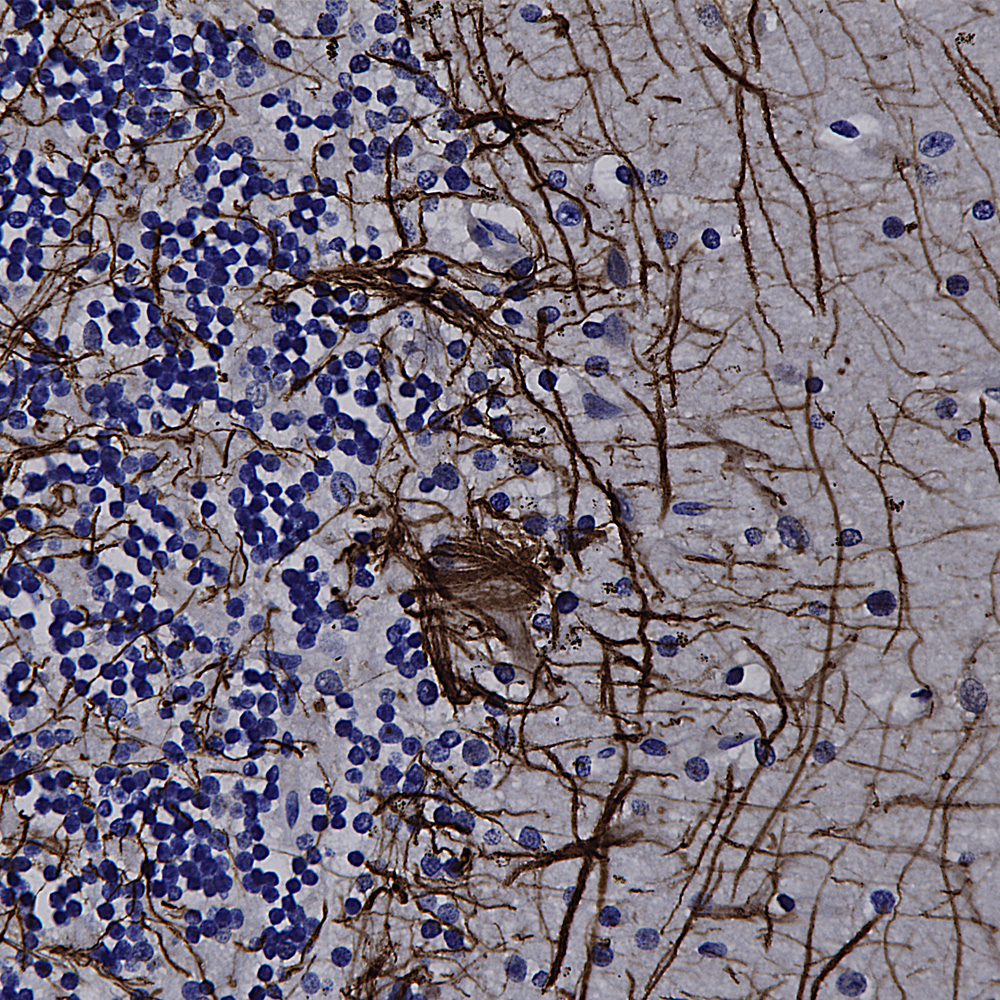
Our recent work on the Uman NF-L antibodies published in pre-print form in BioRχiv was cited for the first time, in JAMA (the Journal of the American Medical Association), only the world’s highest impact medical journal! For a download see here. This work is now published in peer reviewed and freely downloadable form from the journal Brain Communications (https://doi.org/10.1093/braincomms/fcad067). The paper is now also freely downloadable directly from Pubmed https://pubmed.ncbi.nlm.nih.gov/37091583/. We also made a short video Youtube presentation on this work, see here. If you want to check out research utilizing NF-L as a biomarker search for “(NF-L or Nfl or NEFL or “Neurofilament Light”) and biomarker”, over 2,400 publications to date.
We have now tested all of our current mouse monoclonal antibodies on formalin fixed paraffin embedded sections for standard immunohistochemistry (IHC) of rodent and human tissues. As expected, not every antibody worked in this format, though a significant number did. We show images of each antibody which worked and details of how we did the staining. In the near future e will also add a tag to indicated which of our antibodies are not recommended for use in this format. Why did certain antibodies not work? The reason is because antibodies bind to their targets by protein-protein interactions involving individual amino acids. Formalin fixation for IHC is typically quite extensive and works by modifying amino acid residues some of which may be part of a particular antibody binding site. As a result a subset of monoclonal antibodies are expected to have reduced or no ability to bind material processed for IHC. We routinely use antigen retrieval methods as this can in many cases reverse the fixation induced antigenic changes, and details of how we obtained each image are outlined on the web pages. Here are some examples, in each case mouse select the image for a larger view.
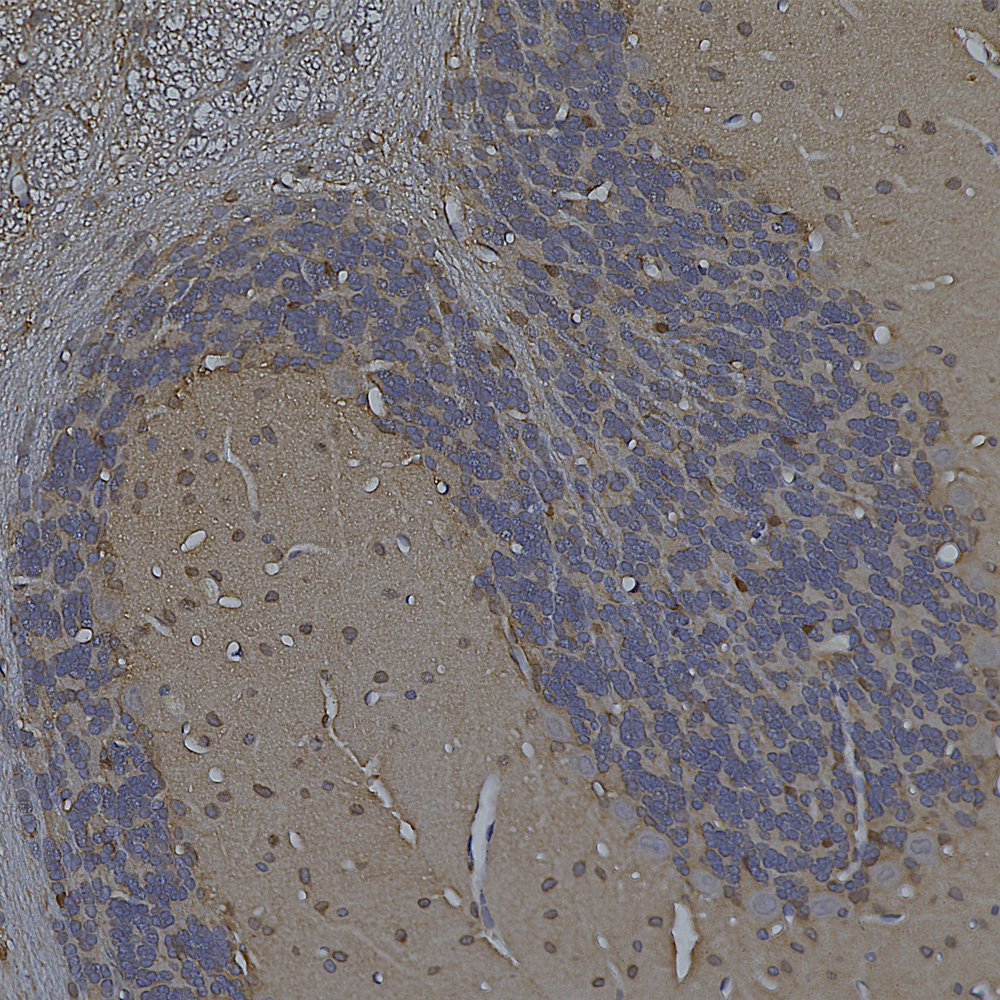
The image below shows a region of human cerebellum stained with MCA-6H112, an epitope mapped antibody raised against the C-terminal peptide of human neurofilament light chain (NF-L) which reveals basket cell processes, parallel fibers and other kinds of axon in both the molecular and granular layers. A version of this image has been posted on Wikipedia for general use, see here.
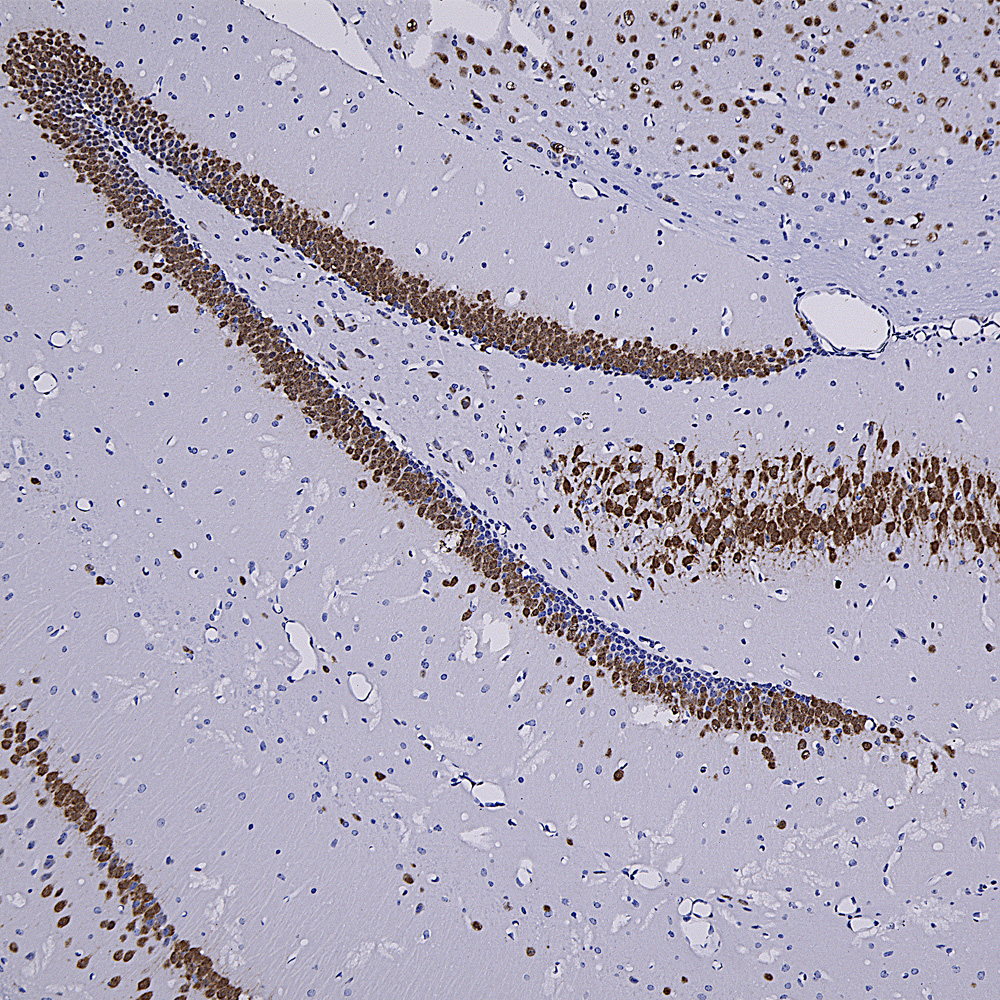
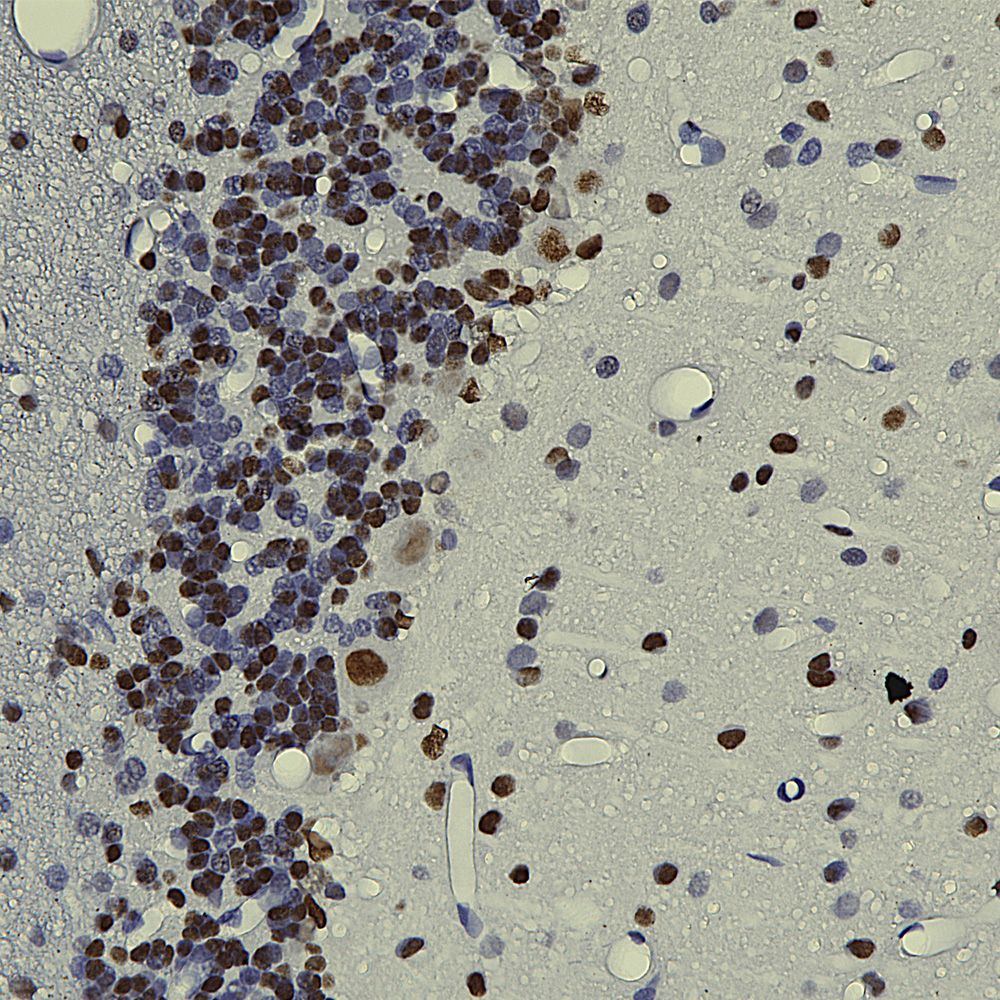
Below is an IHC image of rat cerebellum showing neuronal nuclear staining of with MCA-3H8, our mouse monoclonal antibody to TDP43. TDP43 accumulates in the nuclei of Purkinje cells and other cerebellar neurons.
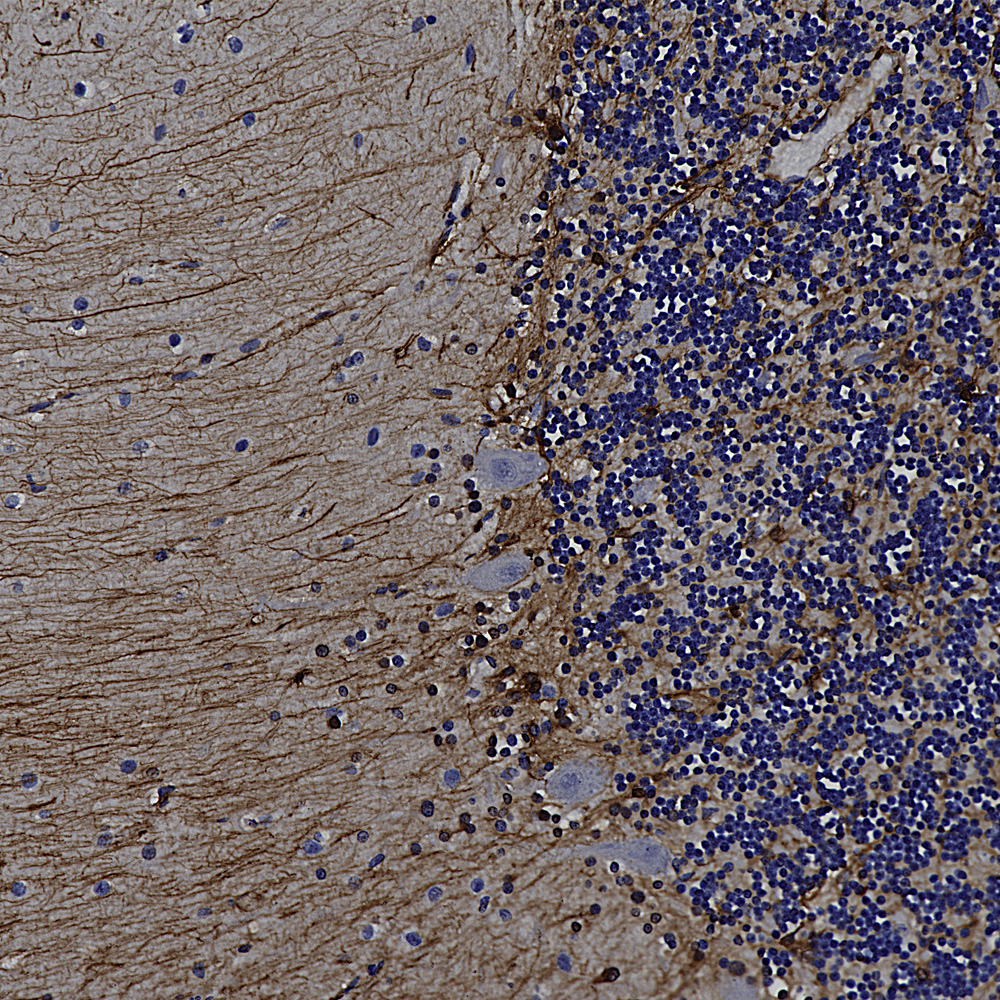
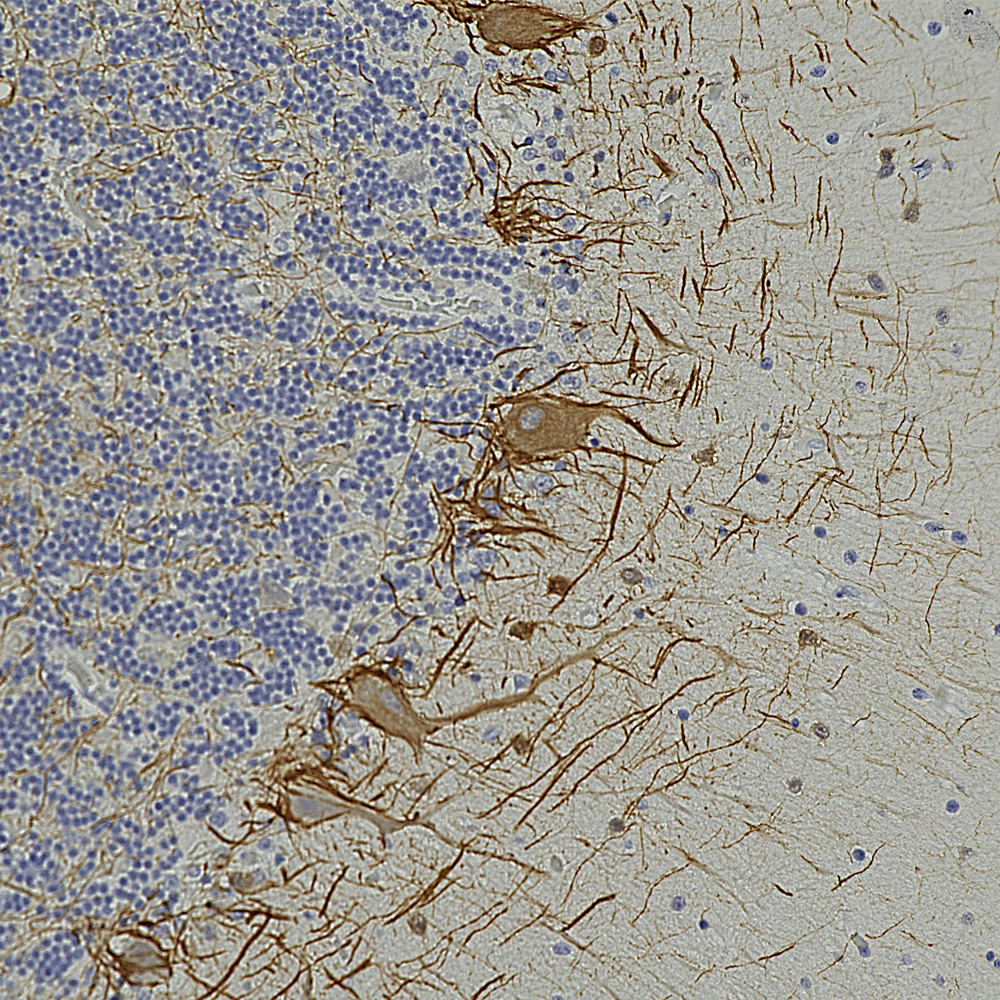
Below is an example of rat cerebellum stained with MCA-3H11, our eoitope mapped mouse monoclonal antibody to neurofilament medium (NF-M), showing basket cell process and parallel fibers.
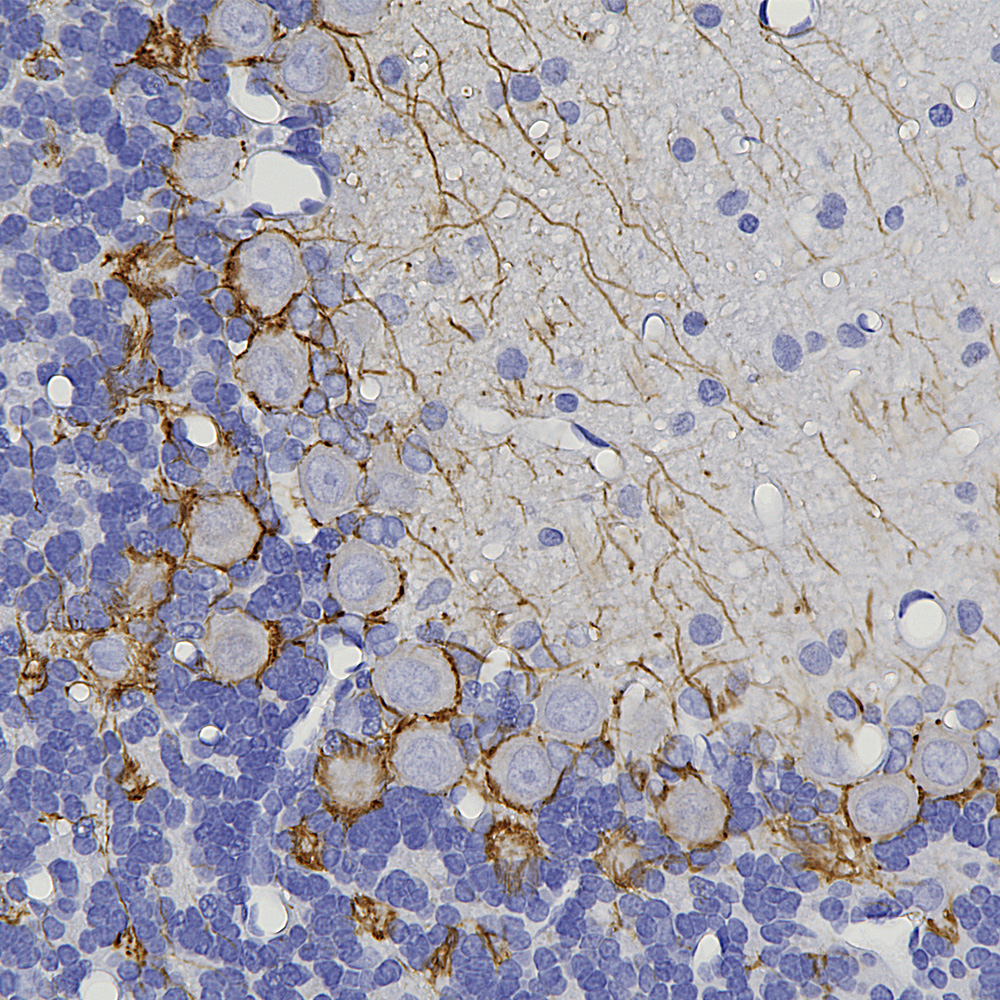
Below is an example of formalin fixed paraffin embedded human cerebral cortex stained with our mouse monoclonal MCA-4A12 to ALDH1-L1, a marker of astrocytes. This antibody produces a different image of astrocytes than that revealed by GFAP antibodies, since ALDH1-L1 is found throughout the cytoplasm of astrocytes while GFAP reveals the cytoskeletal structural core of these cells.
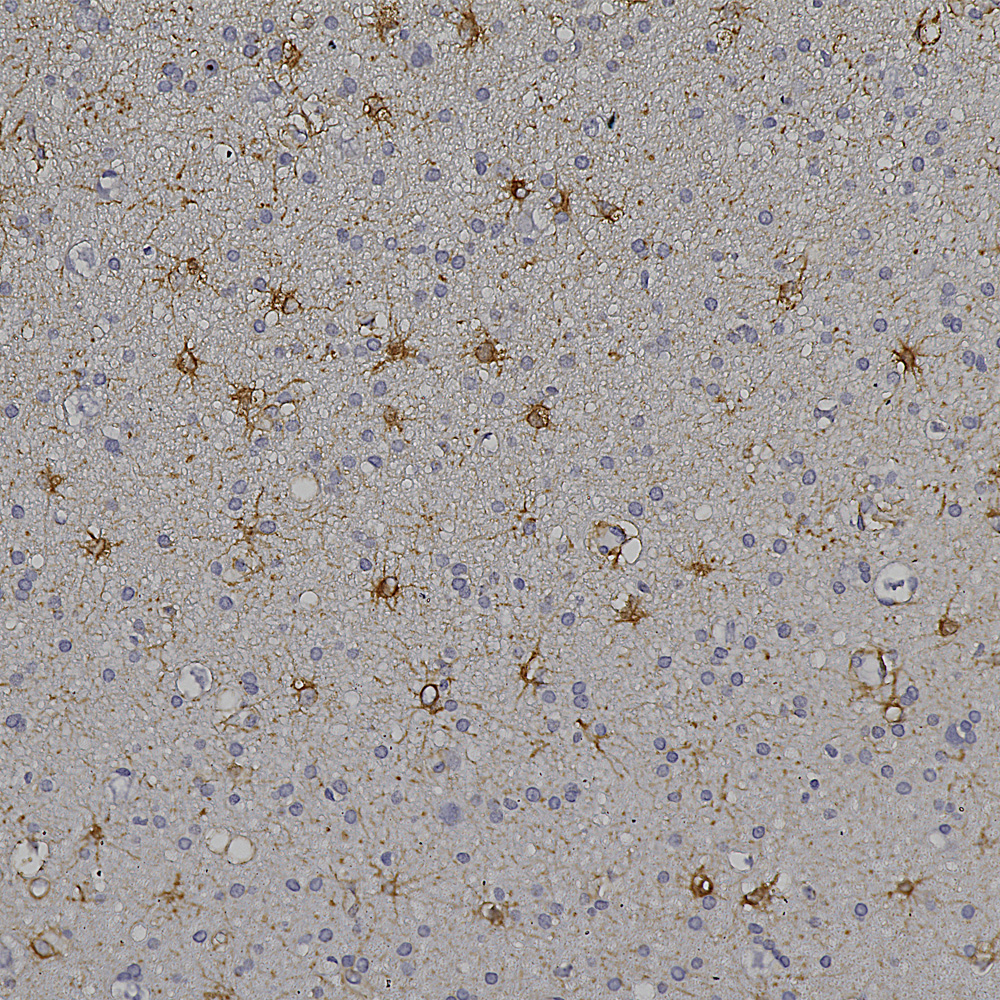
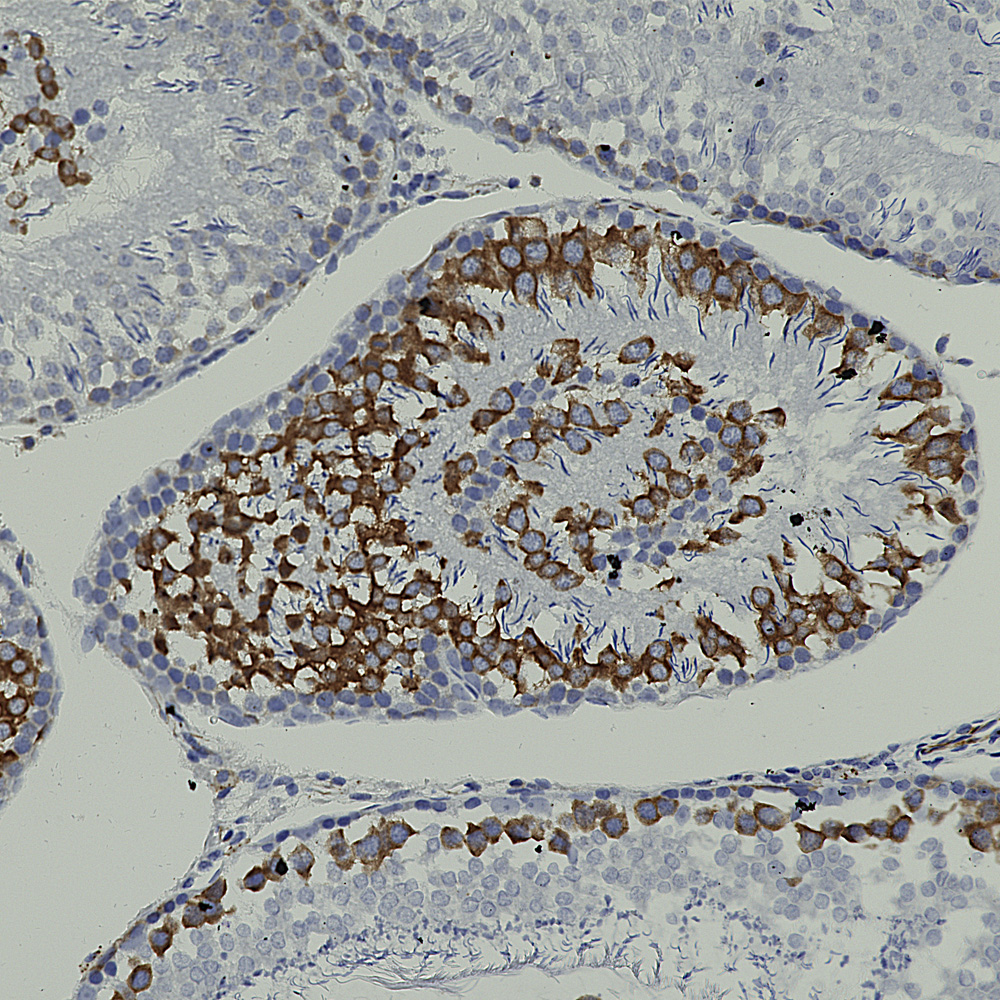
And below is an example of IHC of rat testis stained with our antibody to Nestin, MCA-4D11.
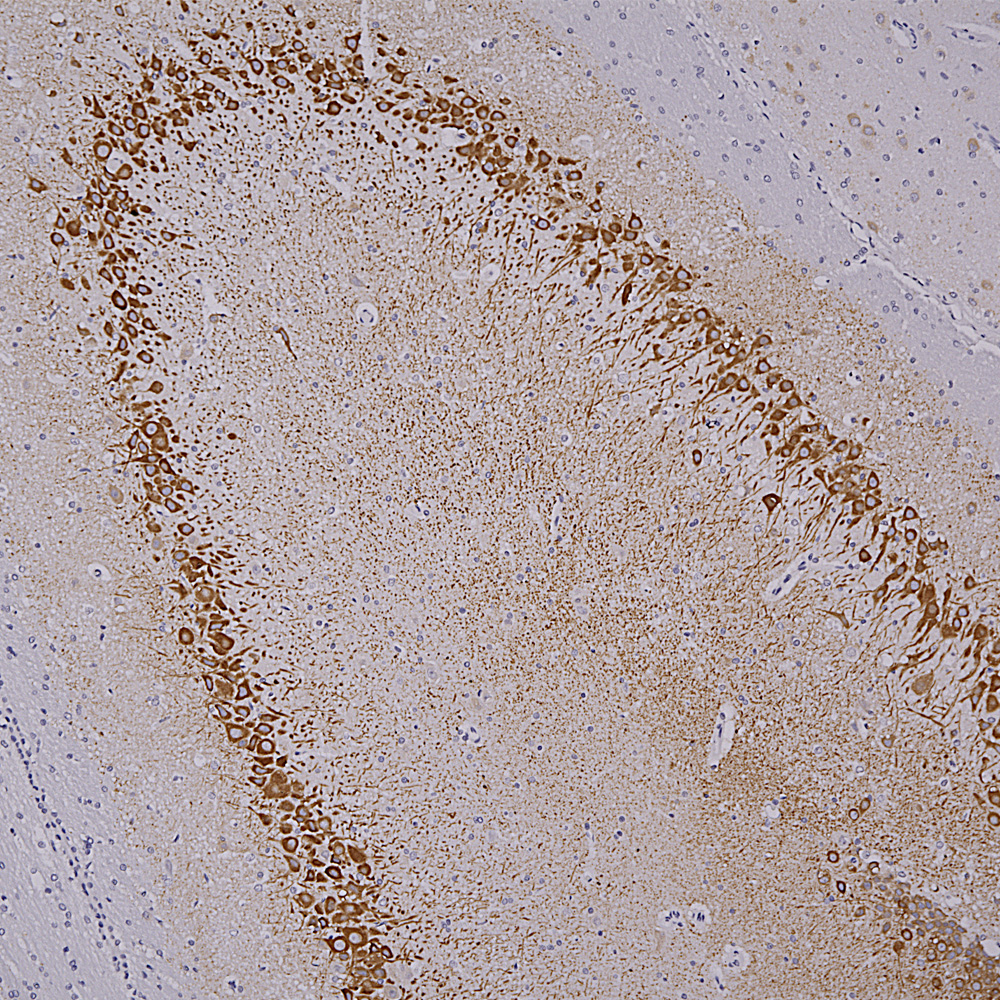
Below is rat hippocampus stained with MCA-4H5 one of our MAP2 antibodies showing staining for hippocampal pyramidal cells.
Below is an IHC specimen of human cerebellum stained with MCA-4H7 mouse monoclonal antibody to calbindin which reveals Purkinke cell dendrites in the molecular layer.
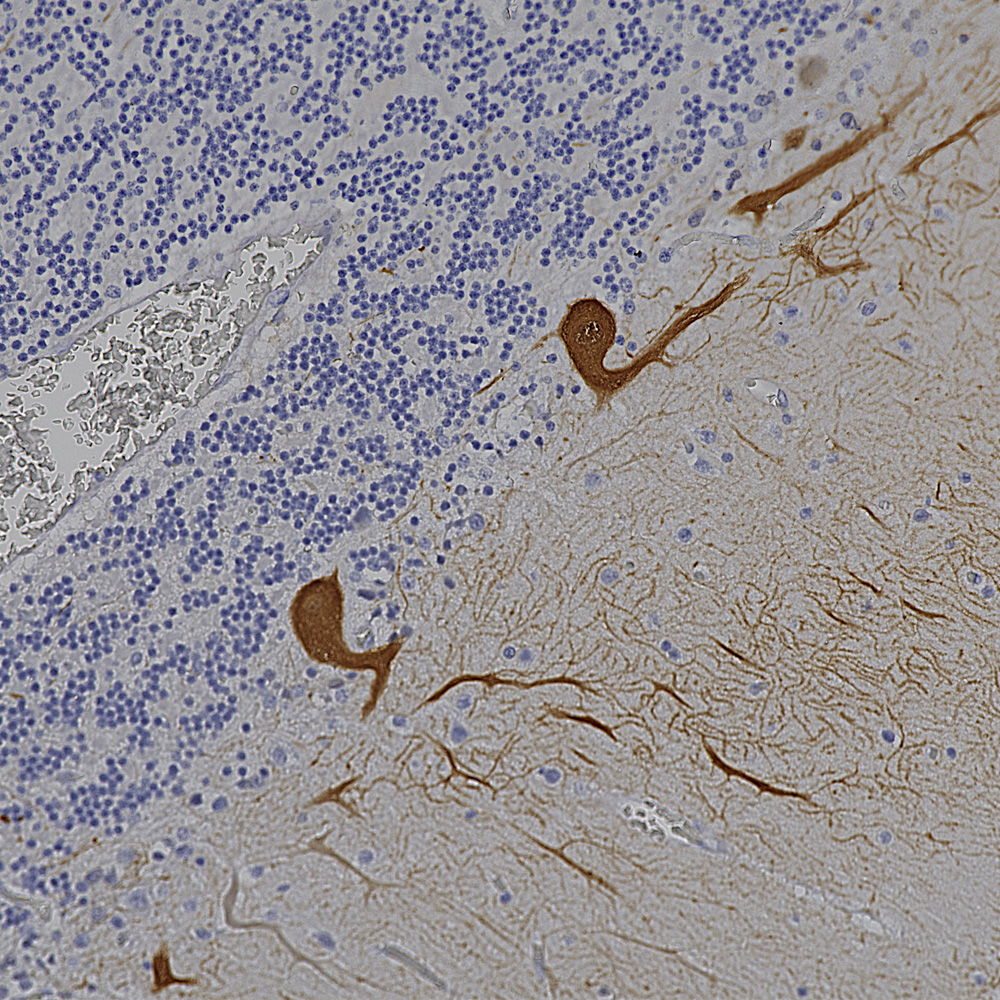
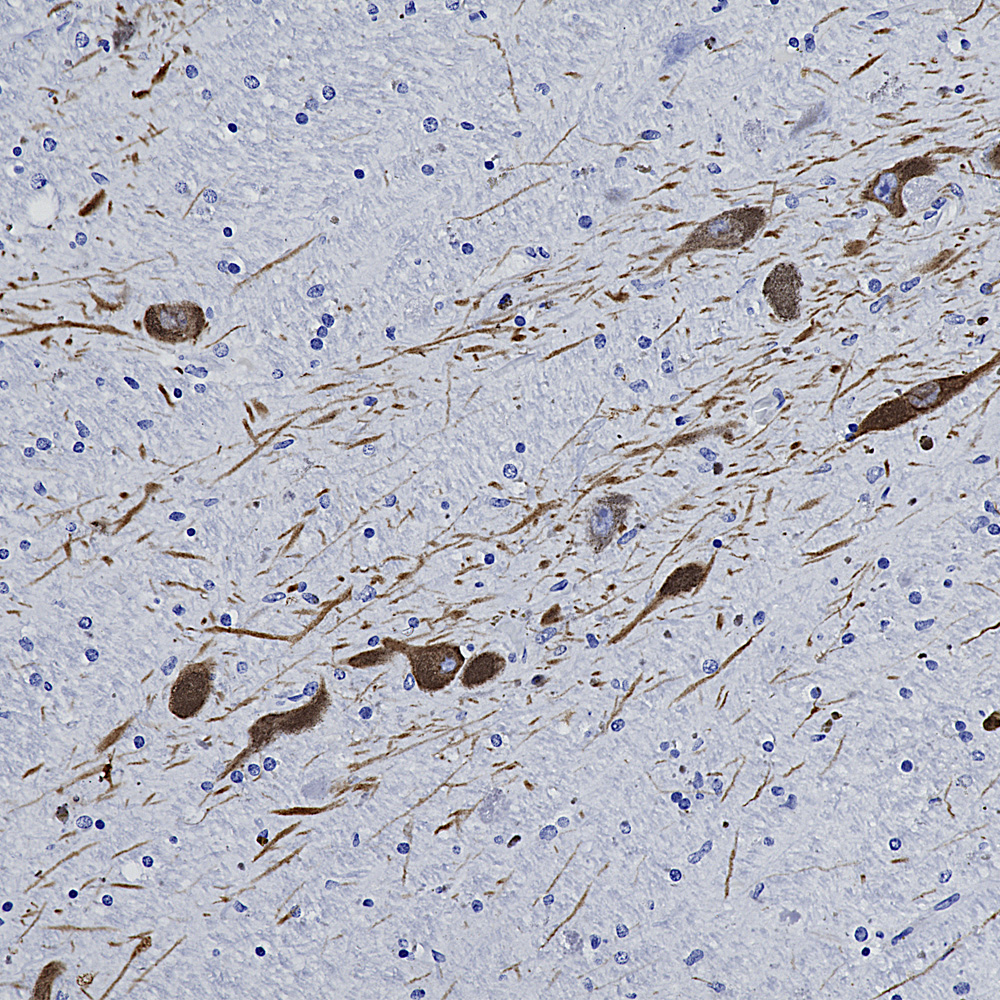
Below is an example of IHC staining with MCA-5B10, our epitope mapped monoclonal antibody to MAP-τ showing flame shaped tangles in the hippocampus of an Alzheimer’s disease patient.
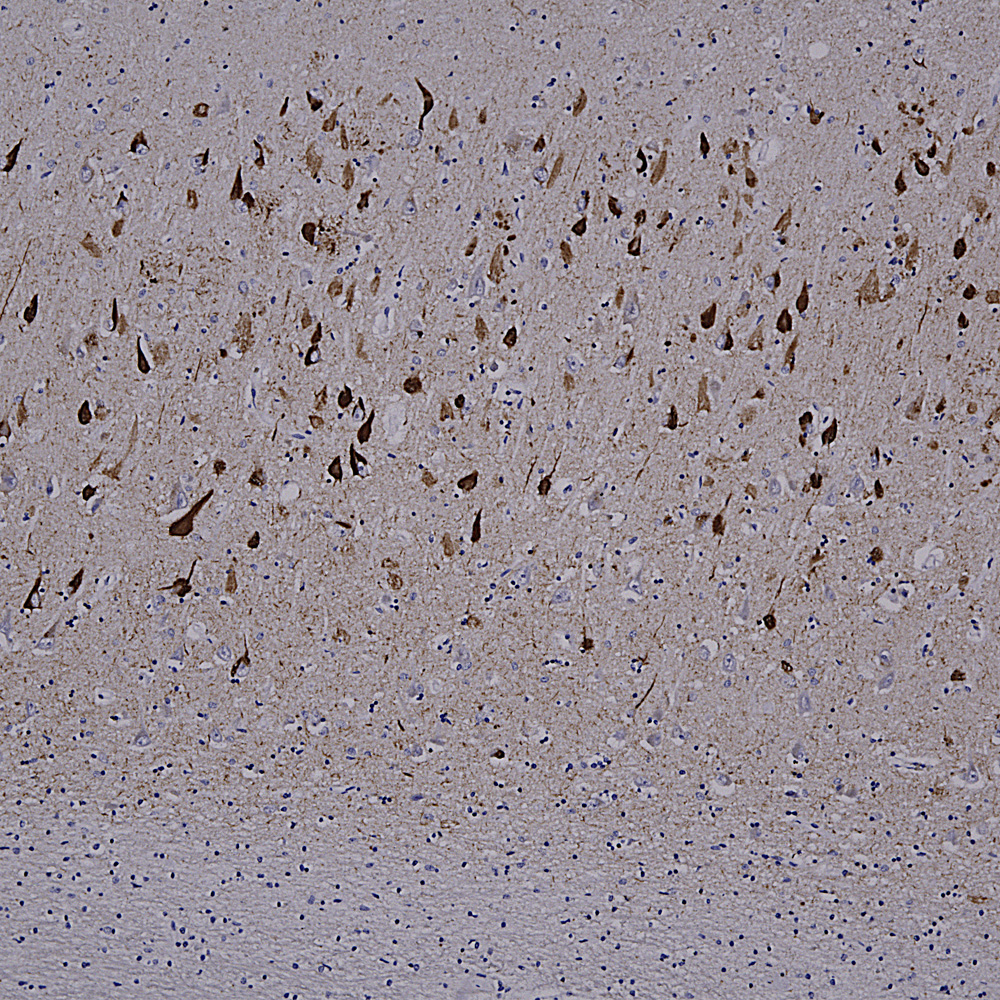
Below is a section of rat kidney stained with MCA-5C21, our monoclonal antibody to Galectin 3.
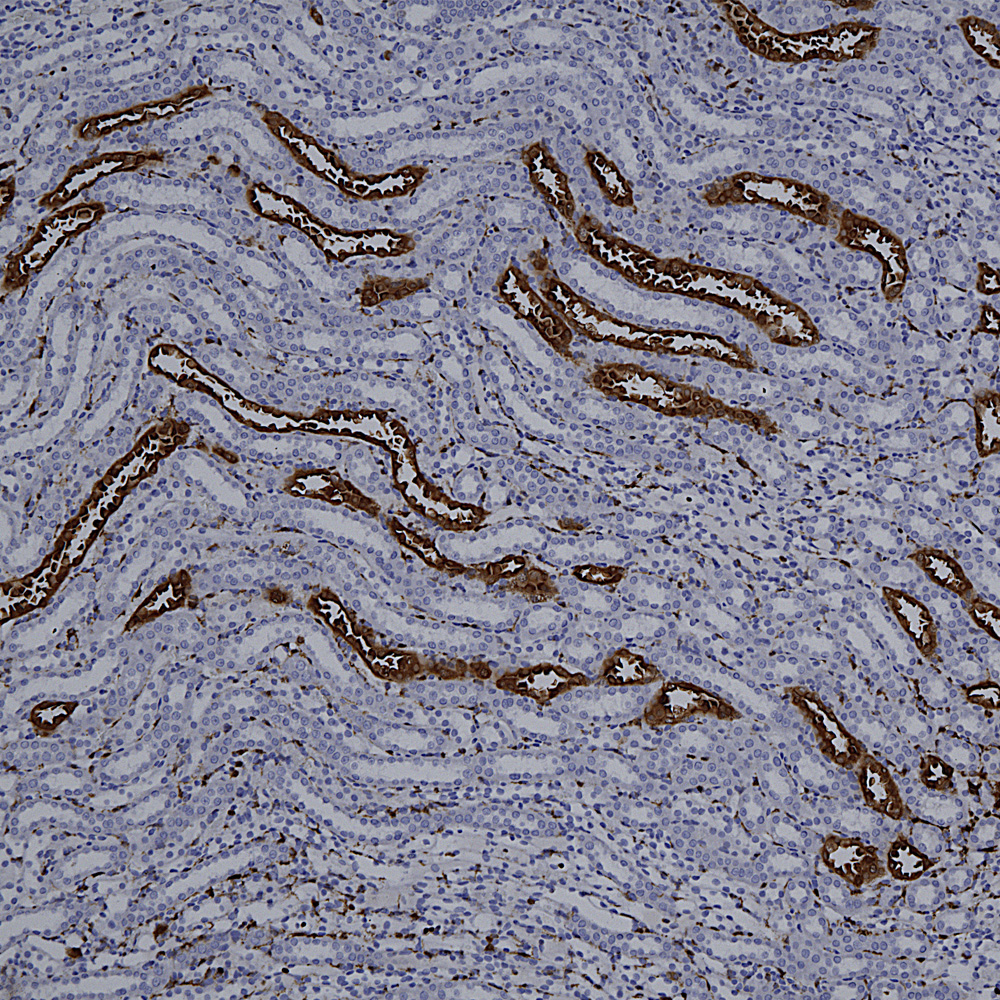
Below is IHC of rat cerebellum stained with our monoclonal antibody to MAP2 MCA-5H11, showing prominent Purkinje cell bodies and dendrites in the molecular layer.
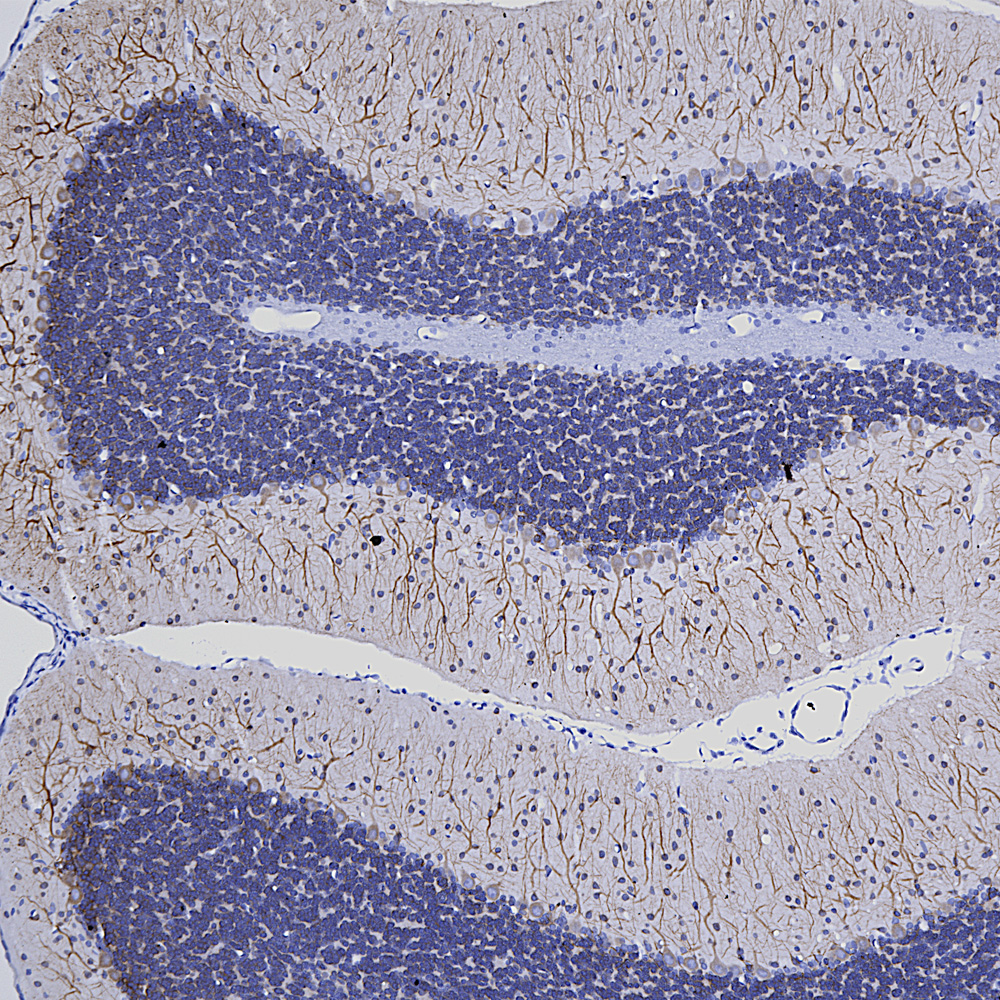
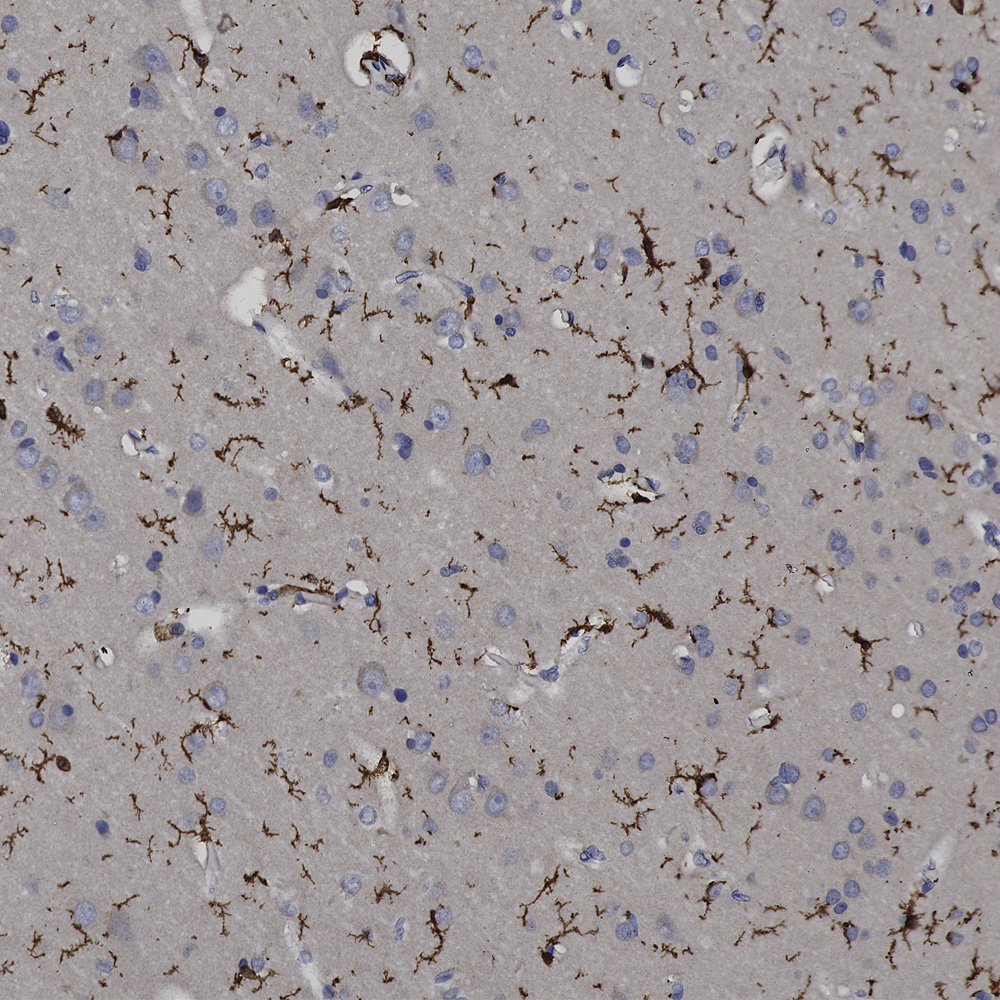
Finally here is IHC of rat cerebellum stained with our MCA-253 monoclonal to non-neuronal enolase, also known as Eno1 or α-enolase, which stains astrocytes and other non-neuronal cells.