EnCor Biotechnology
Mouse Monoclonal Antibody to Aldolases A, B and C (ALDOA, ALDOB, ALDOC or ALDA, ALDB, ALDC, Zebrin II), Cat# MCA-E9
Description
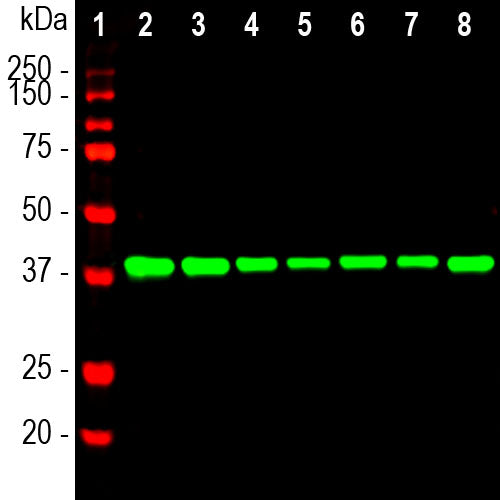
The MCA-E9 antibody was made against recombinant full length aldolase C. Since the three aldolase enzymes are quite similar in amino acid sequence many available antibodies to one protein often have undocumented cross-reactivity with the other two. We used appropriate constructs to shown that MCA-E9 binds to all three aldolase enzymes with equal specificity. We then used nested 20 amino acid peptides to map the MCA-E9 epitope to the peptide YKKDGADFAKWRCVLKISER, amino acids 138-157 of the human aldolase C sequence in 1XFB_A. As expected from the observed cross reactivity this region is quite conserved between the three aldolases (see here). The antibody works well on western blots and for IF but is not recommended for ICC and IHC. An alternative mouse antibody to aldolase C is MCA-4A9, which is specific for aldolase C, and which works well for ICC and IHC. A third EnCor mouse monoclonal antibody MCA-1A1, binds with the C-terminal peptide, also more variable between the three aldolases.
Add a short description for this tabbed section
| Immunogen: | Recombinant human full-length aldolase C |
| HGNC Name: | ALDOC |
| UniProt: | P09972, P05062, P04075 |
| Molecular Weight: | 40kDa |
| Host: | Mouse |
| Isotype: | IgG1 |
| Species Cross-Reactivity: | Human, rat, mouse |
| RRID: | AB_2572223 |
| Format: | Protein G affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, ICC. IF/IHC not recommended |
| Recommended Dilutions: | WB: 1:10,000. ICC: 1:1,000 |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
Aldolases are important glycolytic cytosolic enzymes which catalyse the reversible conversion of fructose-1,6-bisphosphate to glyceraldehyde 3-phosphate. There are three aldolase isozymes coded by three distinct genes in mammals, namely aldolases A, B, and C. Aldolase A is heavily expressed in muscle enzyme and aldolase B is heavily expressed in liver and is regarded as a liver specific enzyme (1-5). In the adult, aldolase C is the brain-specific isozyme expressed in astrocytes and a few classes of neurons, notably Purkinje cells (4). Appropriate antibodies to aldolase C are therefore useful to identify astrocytes in cell culture and sectioned material, and the enzyme may be over expressed in some forms of cancer (6). Recent studies also suggest that detection of aldolase C in blood may be a useful biomarker of the severity and recovery process in patients suffering from the sequelae of traumatic brain injury (7).
This antibody has been tested on formalin fixed and paraffin embedded samples for ICC and IHC, and is not recommended for this purpose. To detect aldolase C by ICC and IHC we recommend an alternate EnCor antibody, MCA-4A9.
1. Popovici T, et al. Localization of aldolase C mRNA in brain cells. FEBS Lett. 268:189-193 (1990).
2. Weber A, et al. Dietary Control of Aldolase B and L-type Pyruvate Kinamse RNAs in Rat. J. Biol. Chem. 259:1798-802 (1984).
3. Mukai T, et al. The structure of the brain-specific rat aldolase C gene and its regional expression. BBRC 174:1035-42 (1991).
4. Royds J, et al. Monoclonal antibody to aldolase C: a selective marker for Purkinje cells in the human cerebellum. Neuropathol. Appl. Neurobiol. 13:11-21 (1987).
5. Thompson R., Kynoch P. Willson V. Cellular localization of aldolase C subunits in human brain. Brain Res. 232:489-93 (1982).
6. Schapira F, Reuber M, Hatzfeld A. Resurgence of two fetal-type of aldolases (A and C) in some fast-growing hepatomas. BBRC 40:321-27 (1970).
7. Arai Y, et al. Position-independent, high-level, and correct regional expression of the rat aldolase C gene in the central nervous system of transgenic mice. Eur. J. Biochem. 221:253-260 (1994).
8. Wachsmuth E, Thorner M, Pfleiderer G. The cellular distribution of aldolase isozymes in rat kidney and brain determined in tissue sections by the immuno-histochemical method. Histochem. 45:143-61 (1975).
9. Walther EU, et al. Genomic sequences of aldolase C (Zebrin II) direct lacZ expression exclusively in non-neuronal cells of transgenic mice. PNAS 95:2615-20 (1998).
Add a short description for this tabbed section