EnCor Biotechnology
Rabbit Polyclonal Antibody to Green Fluorescent Protein (GFP), Cat# RPCA-GFP
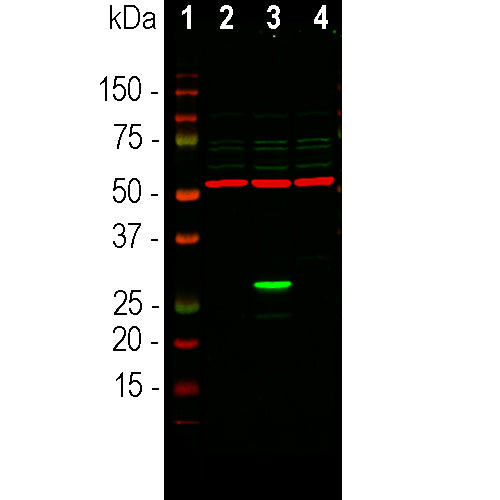
Description
The RPCA-GFP antibody was made against a recombinant GFP construct originating from an Aequoria species which was engineered to improve spectral properties and prevent oligomerization (10). This form of GFP, referred to as AcGFP, is 94% identical to the eGFP developed by Tsien and coworkers and is the form of GFP inserted in the Clontech/Takara pAcGFP and related expression vectors. We also supply the immunogen, PROT-AcGFP. The antibody can be used to verify the expression, size and stability of both AcGFP and eGFP fusion proteins in western blotting experiments and to amplify GFP signals in tissues of transgenic animals. We also supply goat and chicken polyclonal antibodies to this protein, GPCA-GFP and CPCA-GFP.
- Fluorescent Protein Antibodies
- Immunohistochemistry Verified
- New Antibodies
- Our Most Widely Used Reagents
- Rabbit Polyclonal Antibodies
Add a short description for this tabbed section
| Immunogen: | Recombinant AcGFP expressed in and purified from E. coli |
| UniProt: | Q6YGZ0 |
| Molecular Weight: | ~27kDa |
| Host: | Rabbit |
| Species Cross-Reactivity: | N.A. |
| RRID: | AB_2572327 |
| Format: | Immunogen affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:1,000-5,000. ICC: 1:2,000. IF/IHC: 1:2,000-5,000. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
The green fluorescent protein (GFP) is a 27kDa protein isolated originally from the jellyfish Aequoria victoria. It has an endogenous fluorochrome activity with excitation maximum at 395nm and emission maximum at 509nm, which is similar to that of fluorescein (1,2). The GFP gene was sequenced and the origin of the fluorochrome by autocatalytic activity of certain amino acids was discovered (3,4). Much interest in GFP was generated when it was shown that fluorescence develops rapidly when the protein is expressed and requires only molecular oxygen and no other cofactors. As a result GFP can be expressed in fluorescent form in essentially any prokaryotic or eukaryotic cell (5). GFP has been engineered to produce a vast number of variously colored mutants including blue, cyan and yellow protein derivatives, BFP, CFP and YFP (6-9). GFP and other fluorescent proteins derived from other Cnidarians (jellyfish, coral and medusa) are widely used as tracers in transfection and transgenic experiments to monitor gene expression and protein localization in vivo and in in vitro. The crystal structure of GFP was determined (7) which allowed amino acid modifications to improve spectral properties and prevent multimerization (8,9). GFP was the basis of the 2008 Nobel prize in chemistry, specifically “for the discovery and development of the green fluorescent protein, GFP”

Chromogenic immunostaining of formalin fixed paraffin embedded section of GFP injected mouse brain with rabbit pAb to GFP, RPCA-GFP, dilution 1:2,000, detected with DAB (brown) using the Vector Labs ImmPRESS method and reagents with citrate buffer retrieval. Hematoxylin (blue) was used as the counterstain. RPCA-GFP specifically detected GFP at the injection site. Mouse select image for larger view.

Section of mouse tissue expressing GFP in neuronal cells. Panel A shows staining of RPCA-GFP in red in a region containing GFP transfected neurons. Panel B shows only the green GFP signal while Panel C shows only the red antibody signal. Clearly the antibody efficiently detects the transgenic expression of GFP.
We used AcGFP as immunogen for this antibody, sequence taken from AY233272, download here
1 MVSKGAELFT GIVPILIELN GDVNGHKFSV SGEGEGDATY GKLTLKFICT TGKLPVPWPT
61 LVTTLSYGVQ CFSRYPDHMK QHDFFKSAMP EGYIQERTIF FEDDGNYKSR AEVKFEGDTL
121 VNRIELTGTD FKEDGNILGN KMEYNYNAHN VYIMTDKAKN GIKVNFKIRH NIEDGSVQLA
181 DHYQQNTPIG DGPVLLPDNH YLSTQSALSK DPNEKRDHMI YFGFVTAAAI THGMDELYK
This construct is available as PROT-r-AcGFP
1. Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 3:223–39 (1962).
2. Shimomura, O. Structure of the chromophore of Aequorea green fluorescent protein. FEBS Lett. 104:220–2 (1979).
3. Prasher DC, et al. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111:229-33 (1992).
4. Cody CW, et al. Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochem. 32:1212-8 (1993).
5. Chalfie M, et al. Green Fluorescent protein as a marker for gene expression. Science 263:802-5 (1994).
6. Heim R, Prasher DC, Tsien RY. Wavelength mutations and post-translational autoxidation of green fluorescent protein. PNAS 91:12501-04 (1994).
7. Ormo M, et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273:1392-95 (1996).
8. Tsien RY. The green fluorescent protein. Annu. Rev. Biochem. 67:509-44 (1998).
9. Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296:913-6 (2002).
10. Gurskaya NG, et al. A colourless green fluorescent protein homologue from the non-fluorescent hydromedusa Aequorea coerulescens and its fluorescent mutants. Biochem. J. 373:403-8 (2003).
Add a short description for this tabbed section