Frataxin was discovered with defects in the frataxin gene that are causative of Friedreich’s ataxia, a rare progressive neurological disorder (1). The defect is autosomal recessive, meaning that both frataxin genes must be defective in a particular individual in order to generate the disease phenotype. The genetic defects are usually localized to the first intron of the frataxin gene, which in normal individuals includes 7-22 copies of tandemly repeated GAA trinucleotides. In affected individuals, there are many more GAA repeats, 200-900 being noted in the original paper, with most individuals having 700-800 (1). This disease is therefore one of the family of diseases associated with various forms of nucleotide repeat amplification, other well known examples being Huntington’s disease, some forms of ALS and fragile X syndrome. In the case of Friedreich’s ataxia, the repeats result in lowered level of frataxin protein production, and this in turn leads to progressive cell death, particularly in neurons of the spinal cord and peripheral ganglia.
The normal function of frataxin is not well understood, but appears to be involved in protecting mitochondria from free radical damage. In affected individuals, there are motor and sensory deficits and a variety of other problems which may include heart disorders and diabetes. The HGNC name for this protein is FXN.
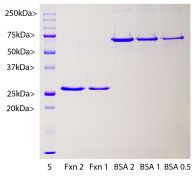
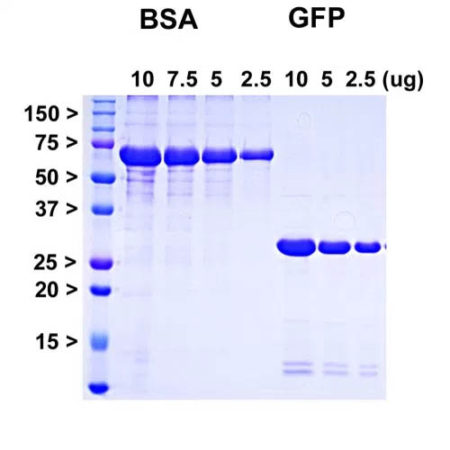
The frataxin pre-protein has an N-terminal leader sequence which is responsible for targeting towards and insertion into the mitochondria. During this process the pre-protein is proteolytically processed resulting in the removal of the N-terminal 55 amino acids. We generated a cDNA encoding the functional mitochondrial form of human frataxin, corresponding to amino acids 56-210 and expressed this in E. coli. The vector adds an N-terminal His-tag which was use to purify the protein and this, along with some other vector derived sequence, adds about 5 kDa to the molecule. The construct therefore has a total size of about 27 kDa as shown: