| Name: | Mouse monoclonal antibody to alpha-II-spectrin |
| Immunogen: | Recombinant C-terminal region of human αII spectrin expressed in and purified from E. coli |
| HGNC Name: | SPTAN1 |
| UniProt: | Q13813 |
| Molecular Weight: | ~240kDa |
| Host: | Mouse |
| Isotype: | IgG1 |
| Species Cross-Reactivity: | Human, Rat, Mouse |
| RRID: | AB_2572381 |
| Format: | Purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:3,000. IF/ICC: 1:500. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. |

Immunofluorescent analysis of cortical neuron-glial cell culture from E20 rat embryos stained with mouse mAb to αII-Spectrin, MCA-3D7, dilution 1:500 in red, and costained with chicken pAb to Microtubule Associated Protein2 (MAP2), CPCA-MAP2, dilution 1:10,000 in green. The blue is Hoechst staining of nuclear DNA. The spectrin antibody stains membranes of neuronal cell body, axons and their dendrites, while MAP2 antibody labels dendrites and perikarya of mature neurons only.
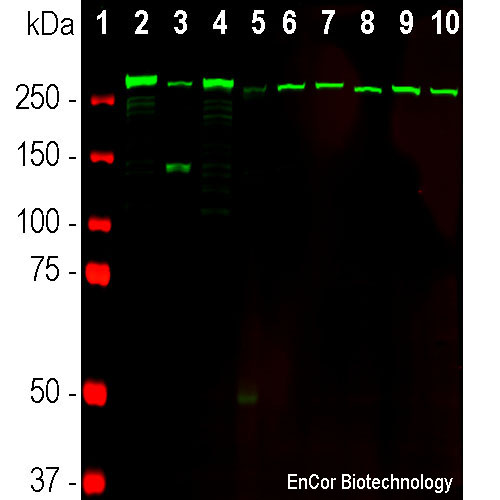
Western blot analysis of different tissue and cell lysates using mouse mAb to αII-specrin, MCA-3D7, dilution 1:2,000 in green: [1] protein standard (in red), [2] rat brain, [3] rat spinal cord, [4] mouse brain, [5] mouse spinal cord, [6] NIH-3T3, [7] HEK293, [8] HeLa, [9] SH-SY5Y, and [10] C6 cells. The prominent band above 250kDa represents the intact αII-spectrin.
Mouse Monoclonal Antibody to αII-Spectrin
Cat# MCA-3D7
$120.00 – $800.00
Spectrin family molecules are important high molecular weight components of the submembranous cytoskeleton of eukaryotic cells. These proteins were isolated originally from lysed red blood cell membrane preparations which were named “ghosts”, which gave rise to the name spectrin (1). Spectrin family molecules are mostly composed of spectrin repeats, compact ~110 amino acid modules made of three closely packed α-helices, though they may also include SH3 domains, PH domains, EF hands and other important binding sites. They function as major components of the membraneous cytoskeleton, mediating interactions between integral membrane proteins, actin and many other cellular components. The MCA-3D7 antibody binds specifically to αII-spectrin, also known as non-erythroid spectrin or fodrin (2-4). In the CNS this protein is expressed only in neurons and so the antibody can be used to reveal the submembranous neuronal cytoskeleton in IF, ICC and IHC. Defects in spectrin genes present as a variety of diseases (5,6). The molecule is subject to proteolysis by calpain producing a 150kDa and 145kDa C-terminal fragments and by caspase producing a slightly different 150kDa C-terminal fragment and a 120kDa C-terminal fragment. Since caspase activation is characteristic of apoptosis and calpain activation of necrosis, it may be possible to use selective monitoring of each type of cell death by monitoring the content of these protein fragments (7).
The MCA-3D7 antibody was made against a recombinant human protein construct derived from the C-terminus of αII-spectrin comprising the C-terminal 2 spectrin repeats, specifically amino acids 2086-2447 from AAB41498.1 expressed in and purified from E. coli. This antibody can be used to study αII-spectrin on western blots and to visualize the neuronal plasma membrane cytoskeleton in cells in culture and sectioned material. Mouse select image at left for larger view.
The spectrin family of proteins was originally discovered as the major components of the
submembranous cytoskeleton of osmotically lysed red blood cells (1). The lysed blood cells could be seen as transparent red blood cell shaped objects in the light microscope and were referred to as red cell “ghosts”. The major proteins of these “ghosts” proved to be actin, ankyrin, band 4.1, and several other proteins including two major bands appearing as 240kDa and 260kDa bands on SDS-PAGE gels. This pair of proteins was named “spectrin” since they were discovered in the red blood cell “ghosts” (1). Later work showed that similar high molecular weight bands were seen in membrane fractions from other eukaryotic cell types.
Work by Levine and Willard described a pair of ~240-260 kDa molecular weight proteins which were
transported at the slowest rate along mammalian axons (2). They named these proteins “fodrin” as antibody studies showed that they were localized in the sheath under the axonal membrane, but not in the core of the axon (fodros means sheath in Greek). Subsequently, fodrin was found to be a member of the spectrin family of proteins, and the spectrin nomenclature is now normally used (3).
Spectrins form tetramers of two α and two β subunits, with the α corresponding to the lower molecular weight ~240 kDa band and the β corresponding to the ~260 kDa or in some case much larger band. Most spectrin tetramers are about 0.2 microns or 200 nm long, and each α and β subunit has a cell type-specific expression pattern. The basic structure of each spectrin subunit is the spectrin repeat, which is a sequence of about 110 amino acids which defines a compact domain containing three closely packed α-helices. Each spectrin subunit contains multiple copies of this repeat, with 20 in each of the α subunits. The β I-IV subunits each contain 17 spectrin repeats, while the β V subunit, also known as β-heavy spectrin, contains 30 of these repeats. The various subunits also contain several other kinds of functional domains, allowing the spectrin tetramer to interact with a variety of protein, ionic, and lipid targets. The α-subunits each contain one calmodulin-like calcium binding region and one Src-homology 3 (SH3) domain, an abundant domain involved in specific protein-protein interactions. The β subunits all have a N-terminal actin-binding domain and may also have one SH3 domain and one pleckstrin homology domain, a multifunctional type of binding domain which in β-I spectrin at least binds the membrane lipid PIP2.
Spectrins are believed to have a function in giving mechanical strength to the plasma membrane since the tetramers associate with each other to form a dense submembranous geodesic meshwork (3). They also bind a variety of other membrane proteins and membrane lipids, and the proteins they bind to are therefore themselves localized in the membrane. Diseases may be associated with defects in one or other of the spectrin subunits (5). For example, some forms of hereditary spherocytosis, the presence of spherical red blood cells which are prone to lysis, can be traced to mutations in some of the spectrin subunits (6). The αII subunit is widely expressed in tissues but, in the nervous system, is found predominantly in neurons. Since the immunogen used to generate the antibody is at the C-terminus of the molecule, the antibody will reveal the 150kDa, 145kDa and 120kDa breakdown products generated by calpain and caspase cleavage on western blots (7). This antibody can also be used to identify neurons and fragments derived from neuronal membranes in cells, in tissue culture, and in sectioned material.
1. Marchesi VT, Steers E. Selective solubilization of a protein component of the red cell membrane. Science 159:203-4 (1968).
2. Levine J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J. Cell Biol. 90:631-42 (1981).
3. Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353-92 (2001).
4. Djinovic-Carugo K, Gautel M, Ylänne J, Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 513:119-23 (2002).
5. Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol. Med. 14:28-36 (2008).
6. Eber S, Lux SE. Hereditary spherocytosis–defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin. Hematol. 41:118-41 (2004).
7. Mondello S, et al. αII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients J. Neurotrauma 27:1203-13 (2010).
Related products
-

Mouse Monoclonal Antibody to α-Synuclein
$120.00 – $800.00
Cat# MCA-2A7Select options This product has multiple variants. The options may be chosen on the product page -

Mouse Monoclonal Antibody to Neurofilament NF-M
$120.00 – $800.00
Cat# MCA-3H11Select options This product has multiple variants. The options may be chosen on the product page -

Mouse Monoclonal Antibody to GAPDH
$120.00 – $800.00
Cat# MCA-1D4Select options This product has multiple variants. The options may be chosen on the product page -

Mouse Monoclonal Antibody to TDP43
$120.00 – $800.00
Cat# MCA-3H8Select options This product has multiple variants. The options may be chosen on the product page
Contact info
EnCor Biotechnology Inc.
4949 SW 41st Boulevard, Ste 40
Gainesville
Florida 32608 USA
Phone: (352) 372 7022
Fax: (352) 372 7066
E-mail: admin@encorbio.com


