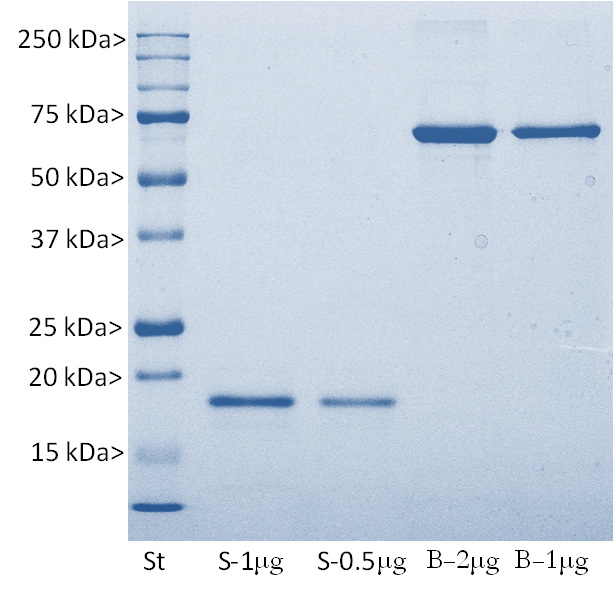
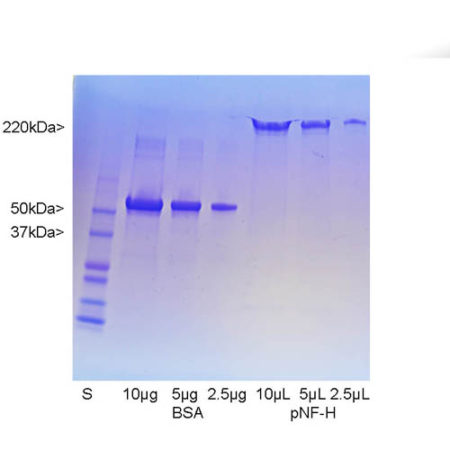
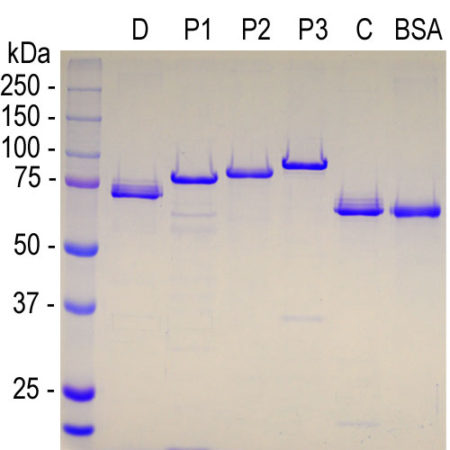
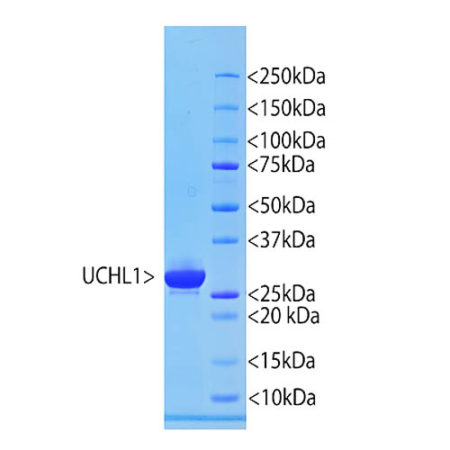
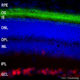
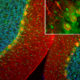
The S100 family of proteins are a large family of Ca2+-binding proteins with 25 members identified in human to date. They are characterized by Ca2+-sites similar to but distinct from the “EF Hand” sequence found in parvalbumin, calmodulin and many other proteins. The term “S100” originated from the finding that this family of proteins has the unusual property of being soluble in 100% saturated ammonium sulfate solution. S100β is a specific member of this protein family and is a 92 amino acid protein, with a molecular weight of 10.7 kDa. It is highly acidic so that 25% of the amino acid composition is aspartic and glutamic acids. S100β is highly conserved across species so that human S100β is 99%, 98%, 100%, 99% and 97% identical to mouse, rat, rabbit, horse and cow S100β, respectively. S100β may form a 21 kDa homodimer containing 4 Ca2+-binding sites. This form can also binds Cu2+ (4 sites) and Zn2+ (6-8 sites) at sites distinct from the Ca2+-binding sites. The two EF-hand like motifs differ in metal binding affinity and are separated by a hinge region with a hydrophobic cleft that is exposed upon Ca2+ binding (1).
S100β is expressed in brain by astrocytes and oligodendrocytes and exerts autocrine and paracrine effects on glia, neurons, and microglia. The protein is also expressed by Schwann cells and in several other tissues including skeletal muscle, aorta, adipose tissue, gut and skin. S100β has been reported to bind to more than 20 intracellular proteins, including several cytoskeletal and filament associated proteins (e. g. tubulin, GFAP, CapZ, desmin, vimentin), transcription factors (e. g. p53), enzymes (e. g. aldolases) and certain protein kinases (e. g. NDR).
Secretion or release of S100β from astrocytes has different effects on cells in a concentration-dependent manner. Nanomolar concentrations of S100β have trophic effects on cells stimulating neurite outgrowth and enhancing survival of neurons during development. On the contrary, micromolar concentrations of S100β are toxic and stimulate the expression of proinflammatory cytokines (e. g. iNOS, COX2, IL1, IL6 and TNFα ) and induce apoptosis.
S100β is also secreted from cells exhibiting extracellular, cytokine-like activities partially via the surface receptor for advanced glycation end products (RAGE) with paracrine effects on neurons. High level expressions of S100β have been linked to carcinogenesis in vitro, particularly melanoma, as well as brain-derived astrocytomas and glioblastomas, where S100β is thought to induce cell proliferation through interaction with p53. Furthermore, elevated levels of S100β were found in blood and CSF of individuals suffering from neuropathological conditions (traumatic brain injury, Creutzfeld-Jakob disease, amyotrophic lateral sclerosis, Alzheimer’s disease, infectious diseases such as bacterial-fungal or lymphocytic meningitis and psychiatric disorders such as schizophrenia and major depressive disorder.
The HGNC name for this protein is S100B.
The S100 family of proteins are a large family of Ca2+-binding proteins with 25 members identified in human to date. They are characterized by Ca2+-sites similar to but distinct from the “EF Hand” sequence found in parvalbumin, calmodulin and many other proteins. The term “S100” originated from the finding that this family of proteins has the unusual property of being soluble in 100% saturated ammonium sulfate solution. S100β is a specific member of this protein family and is a 92 amino acid protein, with a molecular weight of 10.7 kDa. It is highly acidic so that 25% of the amino acid composition is aspartic and glutamic acids. S100β is highly conserved across species so that human S100β is 99%, 98%, 100%, 99% and 97% identical to mouse, rat, rabbit, horse and cow S100β, respectively. S100β may form a 21 kDa homodimer containing 4 Ca2+-binding sites. This form can also binds Cu2+ (4 sites) and Zn2+ (6-8 sites) at sites distinct from the Ca2+-binding sites. The two EF-hand like motifs differ in metal binding affinity and are separated by a hinge region with a hydrophobic cleft that is exposed upon Ca2+ binding (1).
S100β is expressed in brain by astrocytes and oligodendrocytes and exerts autocrine and paracrine effects on glia, neurons, and microglia. The protein is also expressed by Schwann cells and in several other tissues including skeletal muscle, aorta, adipose tissue, gut and skin. S100β has been reported to bind to more than 20 intracellular proteins, including several cytoskeletal and filament associated proteins (e. g. tubulin, GFAP, CapZ, desmin, vimentin), transcription factors (e. g. p53), enzymes (e. g. aldolases) and certain protein kinases (e. g. NDR).
Secretion or release of S100β from astrocytes has different effects on cells in a concentration-dependent manner. Nanomolar concentrations of S100β have trophic effects on cells stimulating neurite outgrowth and enhancing survival of neurons during development. On the contrary, micromolar concentrations of S100β are toxic and stimulate the expression of proinflammatory cytokines (e. g. iNOS, COX2, IL1, IL6 and TNFα ) and induce apoptosis.
S100β is also secreted from cells exhibiting extracellular, cytokine-like activities partially via the surface receptor for advanced glycation end products (RAGE) with paracrine effects on neurons. High level expressions of S100β have been linked to carcinogenesis in vitro, particularly melanoma, as well as brain-derived astrocytomas and glioblastomas, where S100β is thought to induce cell proliferation through interaction with p53. Furthermore, elevated levels of S100β were found in blood and CSF of individuals suffering from neuropathological conditions (traumatic brain injury, Creutzfeld-Jakob disease, amyotrophic lateral sclerosis, Alzheimer’s disease, infectious diseases such as bacterial-fungal or lymphocytic meningitis and psychiatric disorders such as schizophrenia and major depressive disorder.