EnCor Biotechnology
Chicken Polyclonal Antibody to Lamin A/C, Cat# CPCA-LaminAC
Description
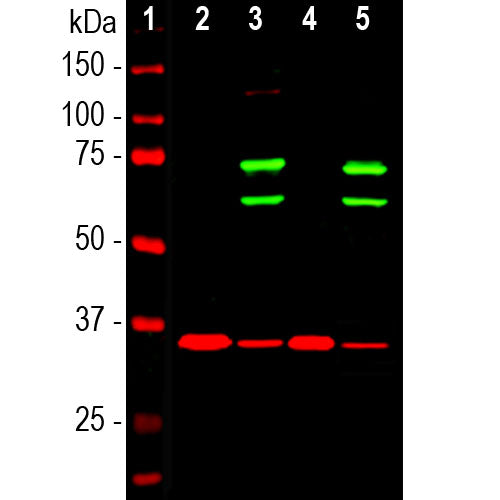
The CPCA-LaminAC was raised against recombinant human lamin A terminating at the C-terminal cleavage site. The antibody also binds lamin C, a slightly larger protein product from the single LMNA gene. We document that the antibody works well not only for western blotting, IF and ICC but also on formalin fixed paraffin embedded IHC sections of human and rodent tissues, select the "Additional Data" for this data. Defects in the LMNA gene are causative of the premature aging of Hutchinson–Gilford progeroid syndrome. The antibody can be used to visualize nuclear lamina in a variety of contexts. We also market a mouse monoclonal antibody to lamin A/C, MCA-4C4.
- Cell Structure Marker
- Chicken Polyclonal Antibodies
- Cytoskeletal Marker
- Immunohistochemistry Verified
Add a short description for this tabbed section
| Immunogen: | Full length recombinant human lamin A protein expressed in and purified from E. coli. |
| HGNC Name: | LMNA |
| UniProt: | P02545 |
| Molecular Weight: | 65kDa and 74kDa |
| Host: | Chicken |
| Species Cross-Reactivity: | Human, Rat, Mouse, Horse, Monkey, Dog |
| RRID: | AB_2572338 |
| Format: | Concentrated IgY preparation plus 0.02% NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:1,000. IF/ICC and IHC: 1:1,000- 1:2,000. |
| Storage: | Store at 4°C. Stable for 12 months from date of receipt. |
Lamin A and lamin C are members of the intermediate filament protein family and are located in the nucleus where they function as skeletal components of the inner nuclear membrane (1). The two proteins are generated by alternate transcription from the single LMNA gene. Lamin A has a molecular weight of about 74kDa while lamin C is 65kDa. The lamin A protein includes a C-terminal segment of 98 amino acids missing from lamin C, while lamin C has a unique C-terminal 6 amino acid peptide not present in lamin A. As a result antibodies raised against lamin A are almost certain bind to lamin C. During cell division the nuclear lamina breaks down and lamin A/C containing filaments depolymerize, this being regulated by phosphorylation by cyclin dependent protein kinase 1. Mutations in the lamin A/C gene are associated with several serious human diseases, including Emery-Dreifuss muscular dystrophy, familial partial lipodystrophy, limb girdle muscular dystrophy, dilated cardiomyopathy, Charcot-Marie-Tooth disease type 2B1, Hutchinson-Gilford progeria syndrome and Hutchinson-Gilford progeria syndrome (3-6).

Chromogenic Immunostaining of a formalin fixed paraffin embedded human cerebral cortex section with chicken pAb to lamin A/C, CPCA-LaminAC, dilution 1:2,000, detected with DAB (brown) following the ABC method with citrate buffer retrieval at pH=6.0. Hematoxylin (blue) was used as the counterstain. Lamin A/C antibody recognizes the nuclear lamina, resulting in a distinct ring pattern. Mouse select image for larger view.
1. Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. PNAS 83:6450-54 (1986).
2. McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 319: 463-8 (1986).
3. Bonne G, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21:285-8 (1999).
4. Novelli G, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am. J. Hum. Genet. 71:426-31 (2002).
5. De Sandre-Giovannoli A, et al. Homozygous Defects In Lmna, Encoding Lamin A/C Nuclear‐Envelope Proteins, Cause Autosomal Recessive Axonal Neuropathy In Human (Charcot‐Marie‐Tooth Disorder Type 2) And Mouse Am. J. Hum. Genet. 70:726-36 (2002).
6. Liu B and Zhou Z. Lamin A/C, laminopathies and premature ageing. Histol. Histopathol. 23:747-63 (2006).
Add a short description for this tabbed section