EnCor Biotechnology
Goat Polyclonal Antibody to Vimentin Cat# GPCA-Vim
Description
The GPCA-Vim antibody can be used to study stem cells and generally to reveal the intermediate filament cytoskeleton. The immunogen used to generate this antibody was full length recombinant human vimentin, PROT-r-Vim, expressed in and purified from E. coli. Vimentin is a major protein of eye lens and cornea, and this mutation renders the molecule unable assemble into normal 10nm filaments. Antibodies to vimentin are useful in studies of stem cells and generally to reveal the filamentous cytoskeleton. The antibody works well on all mammals tested to date for western blotting, IF and ICC, but is not recommended for IHC. It was generated in goat by standard procedures. The same vimentin immunogen was used to produce two high quality epitope mapped monoclonal antibodies to vimentin MCA-2A52 and MCA-2D1, and also widely used rabbit and chicken polyclonal antibodies RPCA-VIM and CPCA-VIM.
- Cell Structure Marker
- Cell Type Marker
- Developmental Marker
- Goat Polyclonal Antibodies
- Not Recommended for IHC
Add a short description for this tabbed section
| Immunogen: | Recombinant human vimentin expressed in and purified from E. coli |
| HGNC Name: | VIM |
| UniProt: | P08670 |
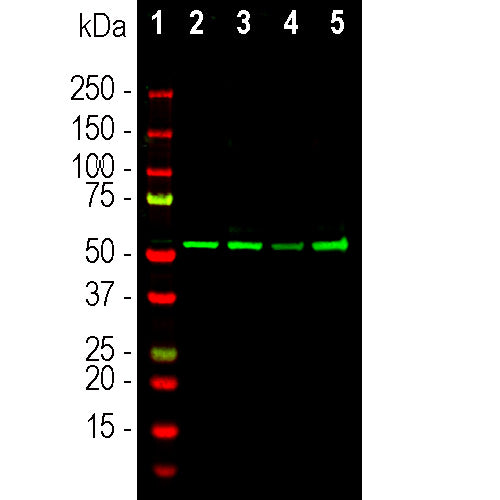
| Molecular Weight: | ~50kDa |
| Host: | Goat |
| Species Cross-Reactivity: | Human, monkey, rat, mouse, cow, pig, horse |
| RRID: | AB_2737582 |
| Format: | Affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, ICC/IF, IHC |
| Recommended Dilutions: | WB: 1:5,000. IF/ICC: 1:500-1,000. IHC: not recommended |
| Storage: | Store at 4°C for short term, for longer term at -20°C |
Vimentin is a protein subunit of the intermediate or 10nm filaments found in the cytoplasm of many cell types (1). Intermediate filaments are relatively stable fibrous components of cells which appear to have primarily a mechanical function. Many cell lines such as HEK293, HeLa, 3T3 and Cos cells contain prominent vimentin networks (1). Vimentin containing filaments accumulate around aggresomes, cytoplasmic clumps of misfolded and often ubiquitinated proteins, and so vimentin antibodies provide one means to identify these structures (2). Vimentin is a major protein of eye lens and cornea, and found in mesenchymal tissues in adult mammals. In the CNS it is found in endothelia and developing neurons, developing and some mature astrocytes, microglia, mature Bergmann glia in the cerebellum, Müller glia in the retina and ependymal cells (e.g. 3,4). Mutations in the vimentin gene may cause cataracts (5,6), and elevated levels of vimentin in blood samples are associated with onset of cancer (7,8). Vimentin levels increase in a variety of cell types as they become cancerous, suggesting that increase in expression of this protein is a useful diagnostic marker of the epithelial-mesenchymal transition, an important step in the metastasis of carcinoma cells (9).
This antibody has been tested on formalin fixed and paraffin embedded samples for IHC, and is not recommended for this purpose.
1. Franke WW, et al. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. PNAS 75:5034–8 (1978).
2. Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143:1883-98 (1998).
3. Dahl D, et al. Vimentin, the 57 000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur. J. Cell Biol. 24:191-6 (1981).
4. Shaw, G. et al. An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur. J. Cell Biol. 26:68-72 (1981).
5. Muller M, et al. Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum. Molec. Genet. 18:1052-7 (2009).
6. Zhai Y, et al. Targeted exome sequencing of congenital cataracts related genes: broadening the mutation spectrum and genotype-phenotype correlations in 27 Chinese Han families. Sci. Rep. 7:1219 (2017).
7. Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 68:3033-46 (2011).
8. Wong KF, Luk JM. Discovery of lamin B1 and vimentin as circulating biomarkers for early hepatocellular carcinoma. Meth. Mol. Biol. 2909:295-310 (2012).
9. Jia X, et al. Vimentin-a potential biomarker for therapeutic efficiency of HAART. Acta Biochim. Biophys. Sin. (Shanghai) 6:1001-6 (2014).
Add a short description for this tabbed section