EnCor Biotechnology
Mouse Monoclonal Antibody to Calreticulin, Cat# MCA-6C6
Description
The MCA-6C6 antibody was raised against the peptide VESGSLEDDWDFLPPKKI, corresponding to amino acids 191-208 of the human calreticulin protein. This peptide includes the LC3 interacting region (LIR) of the molecule, see here for review. The LIR binds to the various proteins of the ATG8 family which are involved in autophagy. The ATG8 family includes certain microtubule associated protein (MAP) light chains, MAP1LC3A, MAP1LC3B, MAP1LC3B2 and MAP1LC3C and also certain GABA receptor associated proteins GABARAP, GABARAPL1, and GABARAPL2. These proteins may bind specifically to certain LIR motif containing proteins.The LIR is centered on a tryptophan residue followed after a space of two variable amino acids by a leucine, referred to as WxxL in the single letter amino acid code. LIR motifs with variants on the amino acids around the WxxL sequence have different binding specificities for the various proteins of the ATG8 family. This antibody is therefore epitope mapped to a functionally important region of calreticulin and may block binding of other molecules to this LIR site (9,11). The antibody works well for western blotting and for IF, ICC and IHC (for IHC see data under "Additional Data" tab).
- Cell Structure Marker
- Cell Type Marker
- Developmental Marker
- Epitope Mapped Antibodies
- Immunohistochemistry Verified
- Mouse Monoclonal Antibodies
Add a short description for this tabbed section
| Immunogen: | Synthetic peptides VESGSLEDDWDFLPPKKI corresponding to amino acids 191-208 of human calreticulin, including the LC3 interacting region or LIR. |
| HGNC Name: | CALR |
| UniProt: | P27797 |
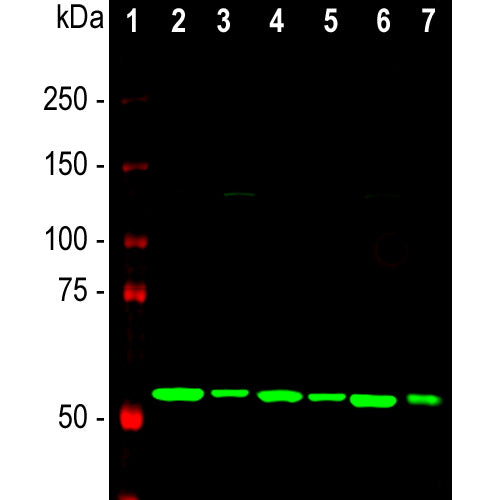
| Molecular Weight: | 48kDa |
| Host: | Mouse |
| Isotype: | IgG1 |
| Species Cross-Reactivity: | Human, Rat, Mouse |
| RRID: | AB_2572240 |
| Format: | Protein G affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:2,000 IF/ICC, and IHC: 1:500-1:1,000. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
Calreticulin was first identified as a calcium binding protein found in rabbit skeletal muscle. It appears to be a multifunctional protein found predominantly in endoplasmic reticulum (ER) and is ubiquitously expressed in a wide range of species (1). The protein has a conserved globular ~170 amino acid lectin-like N-terminal domain, a central proline rich region and a C-terminal low affinity but high capacity acidic Calcium binding region. There is a four-amino acid ER retention sequence (KDEL) at the extreme C-terminus accounting for ER localization. Gene knock out experiments show that the absence of the calreticulin gene is embryonically lethal (2). One of the major functions of calreticulin appears to be buffering Calcium levels in the ER which also affects intracellular calcium signaling (3). Calreticulin also functions as an autophagy receptor and as a molecular chaperone for glycoproteins (4). Calreticulin may also be found on the cell surface where it may mediate phagocytosis of apoptotic or dying tumor cells by triggering immune responses and allowing the immune system to remove tumor cells. This suggests that targeting cell surface calreticulin may be a novel anti-tumor therapy (5-8). The protein includes a functional "LC3 interacting region" or LIR motif (9). Proteins with LIR motifs bind ATG8 family members which in mammals are the LC3 and GABARAP proteins and calreticulin has been shown to bind binds LC3 (10). This binding is one of the essential steps in the process of autophagy, so LIR motif proteins generally function to target other proteins which bind to them for degradation.

Chromogenic immunostaining of a formalin fixed paraffin embedded human brain cortex section with mouse mAb to calreticulin, MCA-6C6, dilution 1:500, detected with DAB (brown) using the the Vector Labs ImmPRESS method and reagents with citrate buffer retrieval. Hematoxylin (blue) was used as the counterstain. In this image, MCA-6C6 intensely labels the cytoplasm of pyramidal neurons. This antibody performs well in testing with both 4% PFA and standard NBF fixed tissues from human and rat. Mouse select image for larger view.

Chromogenic immunostaining of a formalin fixed paraffin embedded human Alzheimer’s disease brain hippocampus section with mouse mAb to calreticulin, MCA-6C6, dilution 1:1,000, detected in DAB (brown) following the Vector Labs ImmPRESS method and reagents with citrate buffer retrieval. In this image, MCA-6C6 intensely labels the cytoplasm of neurons, soe of which have the morphology of tangle bearing cells.
Mouse select image for larger view.
1. Ostwald TJ, MacLennan DH. Isolation of a high affinity calcium-binding protein from sarcoplasmic reticulum. J. Biol. Chem. 249:974-9 (1974).
2. Mesaeli N, et al. Calreticulin is essential for cardiac development. J. Cell Biol. 144:857–68 (1999).
3. Michalak M, et al. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem. J. 417:651–66 (2009).
4. Bedard K, Szabo E, Michalak M, Opas M. Cellular functions of endoplasmic reticulum chaperones calreticulin, calnexin, and ERp57. Int. Rev. Cytol. 245:91–121 (2005).
5. Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13:54–61 (2007).
6. Tesniere A, et al. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 20:504–11 (2008).
7. Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–34 (2005).
8. Fucikova J, et al. Relevance of the chaperone-like protein calreticulin for the biological behavior and clinical outcome of cancer. Immunol. Lett. 193:25-34 (2018).
9. Birgisdottir AB, Lamark T, Johansen T. The LIR motif – crucial for selective autophagy. J. Cell Sci. 126:3237–47 (2013).
10. Yang Y, et al. The ER-localized Ca 2+-binding protein calreticulin couples ER stress to autophagy by associating with microtubule-associated protein 1A/1B light chain 3. J. Biol. Chem. 294:772-782 (2019).
11. Slobodkin MR, Elazar Z. The Atg8 family: multifunctional ubiquitin-like key regulators of autophagy. Essays Biochem. 55:51-64 (2013).
Add a short description for this tabbed section