EnCor Biotechnology
Mouse Monoclonal Antibody to Glial Fibrillary Acidic Protein (GFAP), Cat# MCA-3E10
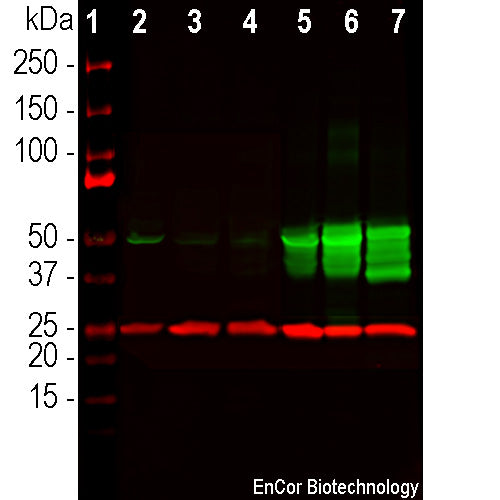
Description
The MCA-3E10 antibody was made against a recombinant human GFAP, construct containing amino acids 71-217 of the human isotype 1 sequence in NP_002046.1. This corresponds to the N-terminal ~50% of the α-helical coiled-coil segment of this molecule. The epitope for this antibody has been mapped to the human peptide EIRFLRKIHEEEVRE (amino acids 196-210) which strongly inhibits binding of the antibody to human GFAP. This peptide is conserved between pig and human GFAP, but that region of the rat and mouse protein are both slightly divergent, having a glutamine instead of the arginine (bold in peptide sequence, red in alignment below). This small divergence is likely the reason for the lower efficiency of this antibody on rodent material. The MCA-3E10 has a KD of 6.157 X 10-10M on the human protein. High quality antibodies to GFAP such as MCA-3E10 are useful for visualizing glia and monitoring developmental, disease and damage related CNS alterations and for ELISA and bead based type assays. It also works well for IHC of human specimens, see data under the "Additional Data" tab. We also produced rabbit, RPCA-GFAP, chicken CPCA-GFAP and goat, GPCA-GFAP polyclonal antibodies to recombinant human GFAP. We also made MCA-5C10, a widely used mouse monoclonal antibody raised against recombinant human GFAP which works equally well for WB, IF/ICC and IHC on material of human, rodent and other mammalian species origin.
- Cell Structure Marker
- Cell Type Marker
- Cytoskeletal Marker
- Developmental Marker
- Immunohistochemistry Verified
- Mouse Monoclonal Antibodies
- Pathology Related Marker
Add a short description for this tabbed section
| Immunogen: | Recombinant human alpha-helical GFAP fragment expressed in and purified from E. coli |
| HGNC Name: | GFAP |
| UniProt: | P14136 |
| Molecular Weight: | 50kDa |
| Host: | Mouse |
| Isotype: | IgG1 |
| Species Cross-Reactivity: | Human, Rat, Mouse, Pig, Cow |
| RRID: | AB_2861215 |
| Format: | Protein G affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:1,000. IF/ICC: 1:500. IHC: 1:1,000-1:2,000. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
Glial fibrillary acidic protein (GFAP) is strongly and specifically expressed in astrocytes, Bergmann glia, certain other glia in the central nervous system, in satellite cells in peripheral ganglia, and in non-myelinating Schwann cells in peripheral nerves. GFAP expression is also seen in developing neural stem cells and GFAP levels may greatly increase in regions of CNS injury or disease. The formation of a GFAP rich "glial scar" following CNS injury may be one reason why reconnection of severed processes is relatively inefficient in adults. Point mutations in the GFAP gene are causative of Alexander disease (5). All forms of Alexander disease are characterized by the presence of Rosenthal fibers, which are GFAP containing cytoplasmic inclusions found in astrocytes. Some interest has recently been focused on GFAP as a protein released into blood and CSF following traumatic brain injury, stroke and other CNS compromises (6,7). Measurement of the levels of blood or CSF GFAP may give information about patient presentation, progress, response to therapy or outcome.
This antibody was made against recombinant human GFAP and works well on human tissues but is less efficient on rodent material. The epitope for this antibody has been mapped to the N-terminal region of the α-helical coiled-coil "rod" region of human GFAP isotype I, and further studies show that the human peptide EIRFLRKIHEEEVRE (196-210) strongly inhibits binding of the antibody to human GFAP. This peptide is conserved between pig and human GFAP, but that of rat and mouse are both slightly divergent, having a glutamine instead of the arginine (see outlined in red below). This small divergence is likely the reason for the lower efficiency of this antibody on rodent material.
Human EIRFLRKIHEEEVRE (196-210)
Rat EIQFLRKIHEEEVRE (194-208)
Mouse EIQFLRKIYEEEVRE (193-207)

Chromogenic immunostaining of a formalin fixed paraffin embedded human brain cortex section with mouse mAb to GFAP, MCA-3E10, dilution 1:1,000, detected with DAB (brown) using the the Vector Labs ImmPRESS method and reagents with citrate buffer retrieval. Hematoxylin (blue) was used as the counterstain. In cortex, GFAP strongly labels astrocytes and glial cells. This antibody performs well in staining with 4% PFA or standard NBF fixed rat and human tissues. MCA-3E10 is our recommended and preferred clone for GFAP paraffin staining applications. Mouse select image for larger view.

Immunofluorescent analysis of mouse hippocampus section stained with mouse mAb to GFAP, MCA-3E10, dilution 1:500 in green, and costained with chicken pAb to NF-L, CPCA-NF-L, dilution 1:2,000, in red. The blue is Hoechst staining of nuclear DNA. Following transcardial perfusion of mouse with 4% paraformaldehyde, brain was post fixed for 24 hours, cut to 45μM, and free-floating sections were stained with the above antibodies. The GFAP antibody stains network of glial cells while the CPCA-NF-L antibody labels axons and dendrites of neuronal cells. While the staining is convincing it is not as strong as seen with other GFAP antibodies, likely due to epitope issue descussed above. So for rodent studies with GFAP antibodies we recommend an alternate mouse monoclonal MCA-5C10 or one of our polyclonal reagents made in rabbit, RPCA-GFAP, chicken CPCA-GFAP or goat, GPCA-GFAP. These were all made against recombinant human GFAP and we document that they work equally well for WB, IF/ICC and IHC on material of human, rodent and other mammalian species origin. Mouse select image for larger view.
1. Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 43:429-35 (1972).
2. Yen SH, Fields KL. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 88:115-26 (1981).
3. Shaw G, Osborn M, Weber K. An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur. J. Cell Biol. 26:68-82 (1981).
4. Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 209:294-301 (2008).
5. Brenner M, et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat. Genet. 27:117-20 2001.
6. Foerch, C. et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. 58:237-45 (2011).
7. Schiff L, Hadker N, Weiser S, Rausch C. A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol. Diagn. Ther. 16:79-92 (2012).
Add a short description for this tabbed section