EnCor Biotechnology
Mouse Monoclonal Antibody to mCherry, Cat# MCA-1C51
Description
The MCA-1C51 antibody was made against full length recombinant mCherry expressed in and purified from E. coli, EnCor product PROT-r-mCherry. The antibody recognizes mCherry strongly on western blots, in appropriate cells and sections and does not react with GFP. It does however react equally well with tdTomato, another derivative of DsRed, see data under the "Additional Data" tab. Our experiments suggest that the epitope is on the outside of the β-barrel structure, between amino acids 121 and 185. MCA-1C51 can be used to verify the size of fusion constructs by western blotting and to amplify the endogenous fluorescence of mCherry in cells and tissues, including formalin fixed paraffin embedded sections, see additional info tab. We used the same recombinant immunogen to produce another mouse mAb, MCA-5A6 and also chicken and goat polyclonals CPCA-mCherry, and GPCA-mCherry respectively.
- Epitope Mapped Antibodies
- Fluorescent Protein Antibodies
- Immunohistochemistry Verified
- Mouse Monoclonal Antibodies
- Our Most Widely Used Reagents
Add a short description for this tabbed section
| Immunogen: | Full length recombinant protein |
| UniProt: | D1MPT3 |
| Molecular Weight: | ~28kDa |
| Host: | Mouse |
| Isotype: | IgG2a heavy, κ light |
| Species Cross-Reactivity: | N.A. |
| RRID: | AB_2572309 |
| Format: | Protein G affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:2,000. IC: 1:500. IF/IHC: 1:2,000-1:5,000. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
The mCherry protein is derived from a natural product, DsRed, originally isolated as a red fluorescent protein from the coral of the genus Discosoma (1). As with other natural fluorescent proteins of Cnidarians (jelly fish, sea anemones, and corals), the natural form of the protein forms stable tetramers in vivo. DsRed was engineered to improve its spectral properties and also prevent multimerization in the Tsien lab, where much work on fluorescent proteins was performed (2). Roger Tsien, along with Martin Chalfie, and Osamyu Shinomura shared the 2008 Nobel prize in chemistry for the discovery and exploitation of Cnidarian fluorescent proteins. Several further cycles of mutation, directed modification and evolutionary selection produced mCherry, which is monomeric and has an excitation maximum at 587nm and emission maximum at 610nm (3). The protein is widely used as a fluorescent tracer in transfection, transgenic, photobleaching and FRET type experiments. The prototype for these fluorescent proteins is Green Fluorescent Protein (GFP), which is a ~27kDa protein isolated originally from the jellyfish Aequoria victoria (4). The mCherry protein is similar in size and general structural properties to GFP (5,6), but, obviously, produces a red rather than a green fluorochrome. As with GFP, mCherry becomes fluorescent due to intrinsic properties requiring only molecular oxygen and so can be readily expressed in a variety of systems.
We used a set of nested 20 amino acid peptides overlapping by 5 amino acids covering the whole of the mCherry sequence as shown below. None of these peptides strongly inhibited binding of MCA-1C11 to recombinant mCherry in a competitive ELISA assay. However four sequential peptides, GEFIYKVKLRGTNFPSDGPV, SDGPVMQKKTMGWEASSERM, SSERMYPEDGALKGEIKQRL
and IKQRLKLKDGGHYDAEVKTT, each showed weak but reproducible inhibition of binding. These peptides are amino acids 121 to 185 of mCherry, corresponding to strands 6 to 11 of the β-barrel structure. Our findings are therefore consistent with MCA-1C11 binding to a discontinuous epitope on the outside face of the mCherry protein.

Chromogenic immunostaining of formalin fixed paraffin embedded mCherry transfected monkey brain with mouse mAb to mCherry, MCA-1C51, dilution 1:2,000, detected with DAB (brown) using the the Vector Labs ImmPRESS method and reagents with citrate buffer retrieval. Hematoxylin (blue) was used as the counterstain. MCA-1C51 specifically detected the soma and axons of mCherry positive neurons in the cortex, as expected for this model. Mouse select image above for larger view.
The sequence of the recombinant mCherry construct used to make this antibody is shown below;
1 MVSKGEEDNM AIIKEFMRFK VHMEGSVNGH EFEIEGEGEG RPYEGTQTAK
51 LKVTKGGPLP FAWDILSPQF MYGSKAYVKH PADIPDYLKL SFPEGFKWER
101 VMNFEDGGVV TVTQDSSLQD GEFIYKVKLR GTNFPSDGPV MQKKTMGWEA
151 SSERMYPEDG ALKGEIKQRL KLKDGGHYDA EVKTTYKAKK PVQLPGAYNV
201 NIKLDITSHN EDYTIVEQYE RAEGRHSTGG MDELYK
The recombinant construct was expressed in and purified from E. coli. The sequence is identical to that found in a series of widely used expression vectors.
 >
>
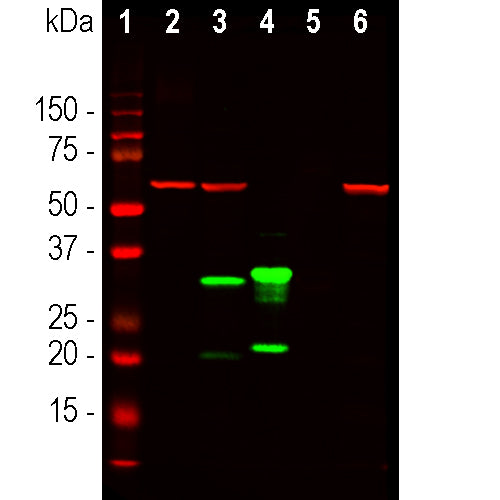
Western blot analysis of HEK293 cell lysates using mouse mAb to mCherry, MCA-1C51, dilution 1:2,000, in green, and rabbit pAb to GAPDH, RPCA-GAPDH, dilution 1:5,000, in red: [1] protein molecular weight standard of indicated molecular weight in kDa, [2] non transfected HEK293 control cells, [3] HEK293 cells transfected with pCI-Neo-mod vector expressing tdTomato protein, [4] HEK293 cells transfected with pCI-Neo-mod vector expressing mCherry-HA protein. †he MCA-1C51 antibody recognizes tdTomato and mCherry proteins revealing major bands at about 60kDa and 30kDa, in green, respectfully. The tdTomato construct tested is identical to that found in several widely used expression vectors and contains two identical fluorescent modules, explaining why it is twice a large as the mCherry construct which contains only one fluorescent module. The red band at 37kDa corresponds to GAPDH protein used as a loading control. Mouse select image for larger view.
1. Matz MV, et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat. Biotechnol. 17:969-73 (1999).
2. Baird GS, Zacharias DA, Tsien RY. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. PNAS 97:11984-9 (2000).
3. Chalfie M, et al. Green fluorescent protein as a marker for gene expression. Science 263:802-5 (1994).
4. Gross LA. et al. The structure of the chromophore within DsRed, a red fluorescent protein from coral. PNAS 97:11990-5 (2000).
5. Heikal AA. et al. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: coral red (dsRed) and yellow (Citrine). PNAS 97:11996-2001 (2000).
6. Shaner NC. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotech. 22:1567-72 (2004).
Add a short description for this tabbed section