EnCor Biotechnology
Chicken Polyclonal Antibody to Calretinin, Cat# CPCA-Calret
Description
The CPCA-Calret antibody was made against full length recombinant human calretinin expressed in and purified from E. coli. The calretinin protein is related in amino acid sequence to calbindin and to a lesser extent parvalbumin, so, for studies of GABAergic interneurons, it is important to verify that antibodies developed against one protein do not cross react with either of the others, which we have done using appropriate recombinant human proteins. We have manufactured mouse monoclonal antibodies to calretinin MCA-3G9 and MCA-6A9, and a rabbit polyclonal RPCA-Calret. We also supply a variety of other mouse, rabbit and chicken antibodies to the related proteins calbindin and parvalbumin allowing double and triple labeling of appropriate cell and tissue samples.
Add a short description for this tabbed section
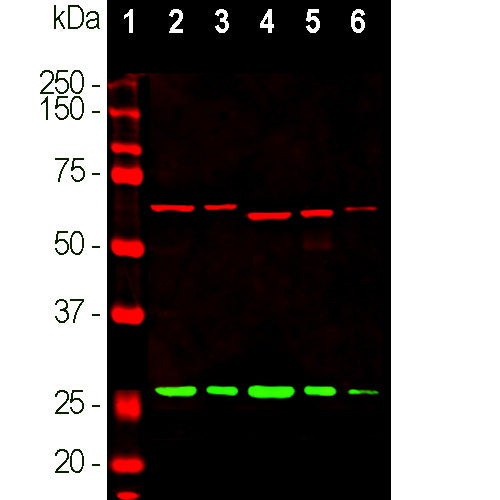
| Immunogen: | Full-length recombinant human calretinin protein expressed in and purified from E. coli. |
| HGNC Name: | CALB2 |
| UniProt: | P22676 |
| Molecular Weight: | 29kDa |
| Host: | Chicken |
| Species Cross-Reactivity: | Human, Rat, Mouse, Cow, Pig, Horse |
| RRID: | AB_2572241 |
| Format: | Concentrated IgY preparation plus 0.02% NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:1,000-1:2,000. ICC/IF or IHC: 1:1,000-1:2,000. |
| Storage: | Store at 4°C. Stable for 12 months from date of receipt. |
Calretinin, as the name suggests, was originally isolated in the retina but was found to be also expressed in mammalian central nerve system, testis, fallopian tube and pancreas (1). It is a cytoplasmic Calcium binding protein which includes typical “EF hand" structures the prototype for which is the protein parvalbumin (2-4). In the brain calretinin it is localized in certain classes of neurons, and antibodies to it are useful for identifying specific neuronal cell types (1). It is particularly concentrated in some cerebellar granular cells and their parallel fibres, but is also found in many GABAergic interneurons in the cortex. These GABAergic interneurons in most cases express only one of three related Calcium binding proteins, namely calretinin, calbindin or parvalbumin (5,6). As a result these important inhibitory interneurons can be identified and classified based on their content of these three proteins. Each type of neuron as defined in this fashion has distinct electrophysiological and functional properties (7). Calretinin deficiency in the mossy cells of the mouse dentate gyrus and granule cells results in abnormal excitability in the cerebellar neuronal network and impairment of long-term potentiation and motor coordination, suggesting that calretinin functions as a general Calcium buffer (8).

Chromogenic Immunostaining of a formalin fixed paraffin embedded rat brain section with chicken pAb to calretinin, CPCA-Calret, dilution 1:2,000, detected with DAB (brown) following the ABC method with citrate buffer retrieval at pH=6.0. Hematoxylin (blue) was used as the counterstain. The subfornical organ region is imaged, showing strong labeling of dorsolateral peripheral neurons (9). Mouse select image for larger view.
1. Rogers JH. Calretinin: a gene for a novel calcium-binding protein expressed principally in neurons. J. Cell Biol. 105:1343-53 (1987).
2. Kretsinger RH, Nockolds CE. Carp Muscle Calcium-binding Protein: II. Structure determination and general description. J. Biol. Chem. 248:3313-26 (1973).
3. Andressen C, Bliimcke I, Celio MR. Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res. 271:181-208 (1993).
4. Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: The role of the calcium binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. The Cerebellum 1:241–58 (2002).
5. Condé F, et al. Local circuit neurons immunoreactive for calretinin, calbindin D‐28k or parvalbumin in monkey prefronatal cortex: Distribution and morphology. J. Comp. Neurol. 341:95-116 (1994).
6. Hof PR, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J. Chem. Neuroanat. 16:77-116 (1999).
7. Bearzatto B, et al. Mono- and dual-frequency fast cerebellar oscillation in mice lacking parvalbumin and/or calbindin D-28k. Eur. J. Neurosci. 22:861-70 (2005).
8. Schiffmann SN, et al. Impaired motor coordination and Purkinje cell excitability in mice lacking calretinin. PNAS 27:5257-62 (1999).
9. Huang S. et al. Electrophysiological properties of rat subfornical organ neurons expressing calbindin D28K. Neurosci. 404:459-469 (1019)..
Add a short description for this tabbed section