EnCor Biotechnology
Mouse Monoclonal Antibody to α-Synuclein, Cat# MCA-2A7
Description
The MCA-2A7 antibody was made against full length recombinant human α-synuclein and recognizes full length human and rodent α-synuclein specifically both in western blots and in immunocytochemical experiments. The epitope for MCA-2A7 is in the region 61-95 which correspond to the “Non-amyloid beta component of Alzheimer’s disease amyloid” (NAC, see above, also see here). The antibody also shows no cross-reactivity with either β or γ-synuclein, see data under the "Additional Data" tab. MCA-2A7 will also bind human α-synuclein containing either the A30P or the A53T Parkinson's associated mutations, see data under the "Additional Info" tab, which also shows the utility of this antibody on IHC of human material.. The antibody has also been used as a capture reagent capable of detecting endogenous α-synuclein in human plasma (8). We also supply a chicken polyclonal antibody made against the same immunogen, CPCA-SNCA.
- Cell Structure Marker
- Cell Type Marker
- Cytoskeletal Marker
- Epitope Mapped Antibodies
- Immunohistochemistry Verified
- Mouse Monoclonal Antibodies
- Pathology Related Marker
Add a short description for this tabbed section
| Immunogen: | Full length human recombinant protein expressed in and purified from E. coli |
| HGNC Name: | SNCA |
| UniProt: | P37840 |
| Molecular Weight: | ~15kDa |
| Host: | Mouse |
| Isotype: | IgG1 heavy, κ light |
| Species Cross-Reactivity: | Human, rat, mouse, cow, pig |
| RRID: | AB_2572383 |
| Format: | Protein G affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, IF/ICC, IHC |
| Recommended Dilutions: | WB: 1:1,000. IF, ICC, and IHC: 1:1,000. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
α-synuclein is a member of the synuclein protein family, the other two members being β and γ-synuclein, each protein is coded for by a distinct but related gene. α-synuclein was originally isolated as a major synaptic vesicle associated protein from the electric organ of the fish Torpedo (1), and direct homologues of α-synuclein are found in all vertebrates. Later work connected α-synuclein expression with several human brain pathologies, it is a major component of the Lewy bodies of Parkinson’s disease (2). Point mutations of α-synuclein proved to be causative of some forms of familial Parkinson’s disease (3-5). One genetic cause of early onset Parkinson’s disease is duplication or triplication of one of the α-synuclein genes leading to excess production of the protein (6,7). α-synuclein is also found in the Lewy bodies of patients with diffuse Lewy body disease and inclusions in glial cells in the brains of patients with multiple system atrophy and amyotrophic lateral sclerosis. α-synuclein is normally heavily expressed in brain and appears to be localized primarily to presynaptic regions, though not with a typical synaptic vesicle distribution pattern.
 .
.
Cortex of a patient with Parkinson's disease (PD) stained with MCA-2A7, 1:1,000 dilution. Antibody revealed with horse radish peroxidase and DAB. The Lewy bodies and other typical inclusions of PD are seen in brown. Mouse select image for larger view.

Chromogenic immunostaining of a NBF fixed paraffin embedded human cerebellum section with mouse mAb to α-synuclein, MCA-2A7, dilution 1:1,000, detected with DAB (brown) using the Vector Labs ImmPRESS method and reagents with citrate buffer retrieval. Hematoxylin (blue) was used as the counterstain. The α-synuclein antibody synaptic regions in the granular and molecular layers of the cerebellum. This antibody performs well in testing with both 4% PFA and standard NBF fixed tissues. Mouse select image for larger view.
 .
.
Immunofluorescent analysis of rat hippocampus section costained with mouse mAb to α-synuclein MCA-2A7, dilution 1:1,000, in green, and chicken pAb to MeCP2 CPCA-MeCP2, dilution 1:2,000 in red). The blue is DAPI staining of nuclear DNA. Following transcardial perfusion of rat with 4% paraformaldehyde, brain was post fixed for 1 hour, cut to 45 μM, and free-floating sections were stained with above antibodies. The α-synuclein protein is concentrated in synaptic regions, and the MeCP2 antibody stains the nuclei of neuronal cells. Mouse select image for larger view.

Immunofluorescent analysis of rat olfactory bulb section costained with mouse mAb to a-synuclein MCA-2A7, dilution 1:1,000 in red, and rabbit pAb to GFAP RPCA-GFAP, dilution 1:5,000 in green. The blue is DAPI staining of nuclear DNA. Following transcardial perfusion of rat with 4% paraformaldehyde, brain was post fixed for 24 hours, cut to 45μM, and free floating sections were stained with above antibodies. The α-synuclein protein is concentrated in synaptic regions, while the GFAP antibody stains the filamentous backbone of astroglial cells. Mouse select image for larger view.

Various truncated and mutant construct were applied to PVDF membranes in about of 400ng of each protein. These were the first 60 amino acids of human α-synuclein , (1-60), amino acids 61 to 140 (61-140), full length α-synuclein but incorporating the A30P and A53T mutations seen associated with familial forms of Parkinson's disease (A30P/A53T), full length α-synuclein but with the central NAC region of amino acids 61-95 missing (d61-95) and finally full length α-synuclein. The strips of PVDF were probed with another EnCor antibody to α-synuclein MCA-3H9, the Santa Cruz monoclonal antibody 211, the Santa Cruz rabbit polyclonal (R-SC) and EnCor's rabbit polyclonal (R-EnCor, RPCA-aSyn). The epitopes for MCA-2A7 and MCA-3H9 are clearly in the NAC region from 61-95, while the 211 antibody epitope is within the C-terminal region from 95-140 (in fact the epitope has been shown to be aminoacids 120-125). Both rabbit antibodies bind constructs including amino acids 61-140, but do not appear to bind amino acids 1-60, suggesting that this region has low immunogenicity. Mouse select image for larger view.

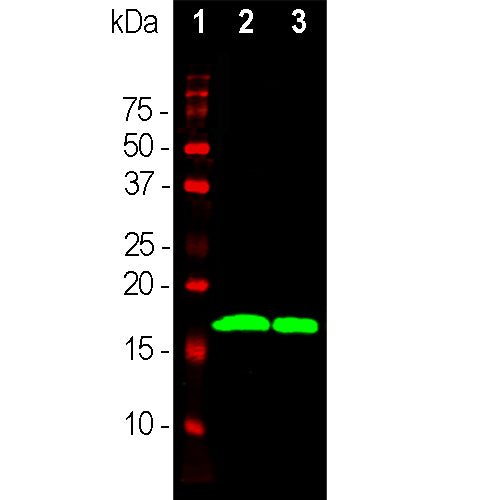
Western (left) and Ponceau S stained blots (right) of recombinant full length human α, β and γ-synuclein in lanes 2, 3 and 4 respectively. MCA-2A7 reacts strongly with α-synuclein and shows no reaction with the other proteins. Lane 1 shows molecular weight standards of indicated molecular weight. Mouse select image for larger view.
Additional References:
A review of the family of intrinsically unstructured proteins, to which the synucleins belong. Dyson, HJ. and Wright, PE. Intrinsically unstructured proteins and their functions. Nature Reviews of Molecular and Cellular Biology 6:197-208 (2005).
A general review of α-synuclein. Cookson MR. Alpha-Synuclein and neuronal cell death. Mol Neurodeg 4:9 (2009).
A paper showing the utility of MCA-2A7 antibody as a potential blood biomarker. Tinsley RB et al. Sensitive and specific detection of α-synuclein in human plasma. J. Neurosci. Res. 88:2693-700 (2010).
1. Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 8:2804-15 (1988).
2. Lavedan C. The Synuclein Family. Genome Research 8:871-80 (1998).
3. Polymeropoulos, MH et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045-7 (1997).
4. Kruger, R et al. Ala30-to-Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nature Genet. 18:106-8 (1998).
5. Chartier-Harlin, M-C. et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167-9 (2004).
6. Singleton, AB.et al. Alpha-synuclein locus triplication causes Parkinson’s disease. Science 302:841 (2003).
7. Ibanez, P. et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 364:1169-71 (2004).
8. Tinsley RD, et al. Sensitive and specific detection of α-synuclein in human plasma. J. Neurosci. Res. 88:2693-700 (2010).
Add a short description for this tabbed section