EnCor Biotechnology
Mouse Monoclonal Antibody to Phospho-MAP-τ-Tp205 (phospho-MAPT T205), Cat# MCA-1H103
Description
The MCA-1H103 antibody was raised against amino acids 190-215 of low molecular weight four tubulin binding repeat τ (aka MAP-tau). This peptide included to known phosphorylation sites phospho-Ser202 and phospho-Thr205 using the sequence in NP_005901.2. This peptide sequence is expressed in all known human τ isotypes and is totally conserved in all mammals, for exact sequence see τ-Epitopes.pdf. The antibody recognizes S202/T205 phosphorylated tau and also T205 phosphoylated tau, but shows no reactivity with non phosphorylated τ only phosphorylated on S202. We have two other mouse monoclonal antibodies binding to other defined epitopes on human τ, MCA-5B10 and MCA-2E9. Both antibodies recognize the unphosphorylated forms of τ and like MCA-1H103 work well on western blots, IF, ICC and IHC.
- Cell Structure Marker
- Cytoskeletal Marker
- Epitope Mapped Antibodies
- Immunohistochemistry Verified
- Mouse Monoclonal Antibodies
- Pathology Related Marker
Add a short description for this tabbed section
| Immunogen: | Peptide sequence 190-215 of the human 4 repeat τ with two phosphate groups on Ser 202 and Thr-205, KSGDRSGYSSPGS(PO4)PGT(PO4)PGSRSRTPSL. Peptide included a C-terminal Cys residue to allow coupling to KLH. |
| HGNC Name: | MAPT |
| UniProt: | P10636 |
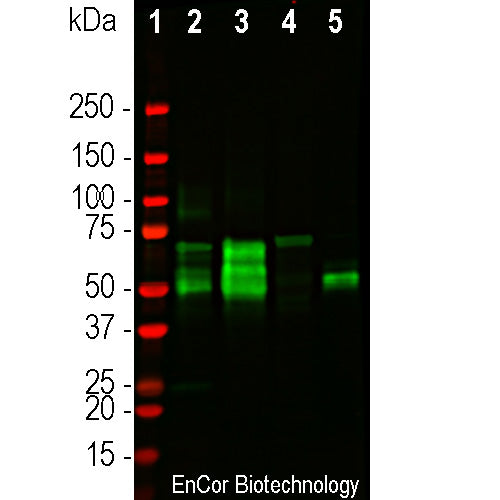
| Molecular Weight: | 48-67 kDa by SDS-PAGE, with higher molecular weight "big tau" isoforms mostly in peripheral tissues. |
| Host: | Mouse |
| Isotype: | IgG1 |
| Species Cross-Reactivity: | Human, Rat, Mouse |
| Format: | Protein G affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, ICC/IF, IHC |
| Recommended Dilutions: | Western blot: 1:10,000. IF/ICC 1:1,000. IHC 1:10,000 |
| Storage: | Stable at 4°C for one year, for longer term store at -20°C |
Tau, aka τ, is a low molecular weight member of the microtubule associated protein or MAP family. Several serious human diseases are associated with accumulations of tau protein, most notably the neurofibrillary tangles of Alzheimer's disease. Accumulations of τ in neurons are also characteristic of chronic traumatic encephalopathy, Pick's disease and several other neurodegenerative diseases. Together these disorders are known as "tauopathies". The single mammalian τ gene produces at least 9 different proteins by alternate transcription. In the central nervous system 6 isoforms ranging from 48-67kDa by SDS-PAGE predominate, though larger isotypes are seen mostly in the peripheral nervous system. The τ molecules are very heavily charged and run on SDS-PAGE much more slowly than predicted from their real molecular size. For example the smallest human τ isotype runs at 48kDa on SDS-PAGE but the real molecular weight is 32kDa. Tau proteins are substrates for ser/thr phosphorylation and other post-translational modifications.

Section of rat hippocampuse stained with MCA-1H103 diluted 1:1,000. and detected with DAB (brown) using the Vector Labs ImmPRESS method and reagents with citra buffer retrieval. The antibody staines neuronal perikarya and processes. We have not yet verified that this antibody works on typical human brain sections for IHC.

Dot blots of 2µL of 10mg/mL peptides based on amino acids 190-215 of the human sequence immobiized on nitrocellulose. Peptides were synthesized with with both S202 and T205 phosphorylated (Ser-P202/Thr-P205), just Threonine 205 phosphorylated (Thr-P205), just S202 phosphorylated (Ser-P202) or niether site phosphorylated as shown. Clearly MCA-1H103 strongly recognizes peptides in which T205 is phosphorylated but shows no reactivity with unphosphorylated or Ser202 phosphorylated tau. Binding of MCA-1H103 is also apparently not affected by the state of phosphorylation of Ser202.
1. Weingarten, M. D., Lockwood, A. H., Hwo, S.-Y. and Kirschner, M. W. A protein factor essential for microtubule assembly. Proc. Nat. Acad. Sci. USA 72:1858–1862 (1975).
2. Cleveland, D. W., Hwo, S. Y., Kirschner, M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 116:207-25 (1977).
3. Goedert, M., Spillantini, M. G. A century of Alzheimer’s disease. Science 314:777–81 (2006).
4. Ballatore, C., Lee, V. M., Trojanowski, J. Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8:663–72 (2007).
5. Wolfe, M. S. Tau mutations in neurodegenerative diseases. J. Biol. Chem. 284:6021-25 (2009).
6. Goedert M, Spillantini MG, Crowther RA. Cloning of a big tau microtubule-associated protein characteristic of the peripheral nervous system. PNAS 89:1983-7 (1992).
7. Boyne LJ, Tessler A, Murray M, Fischer I. Distribution of Big tau in the central nervous system of the adult and developing rat. J. Comp. Neurol. 358:279-93 (1995).
8. Goedert M, et al. Multiple Isoforms of human microtubule-associated protein in tau: Sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron 3:519-526 (1989).
Add a short description for this tabbed section