| Name: | Recombinant Green Fluorescent Protein |
| HGNC Name: | NA |
| RRID: | Pending |
| Format: | 1mg/mL in 6M Urea, !0mM phosphate pH=7.5 |
| Applications: | Protein standard, immunogen |
| Storage: | Store at -20°C |
| Uniprot: | Q6YGZ0 |
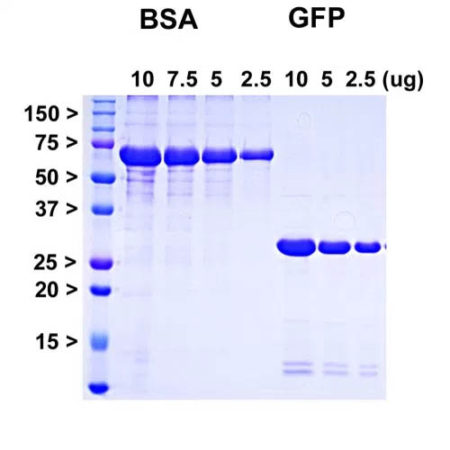
Coomassie brilliant blue stained SDS-PAGE of PROT-AcGFP and appropriate standards. Protein standards of indicated molecular size in kiloDaltons in leftmost lane, next four lanes show indicated microgram amounts of pure bovine serum albumin (BSA), final three lanes show indicated microgram amounts of recombinant AcGFP.
Recombinant Green Fluorescent Protein
Cat# Prot-r-AcGFP
$300.00 – $2,000.00
The green fluorescent protein (GFP) is a 27kDa protein isolated originally from the jellyfish Aequoria victoria. It has an endogenous fluorochrome activity with excitation maximum at 395nm and emission maximum at 509nm, which is similar to that of fluorescein (1,2). The GFP gene was cloned and sequenced and the origin of the fluorochrome by autocatalytic activity of certain amino acids was discovered (3,4). Much interest in GFP was generated when it was shown that fluorescence develops rapidly when the protein is expressed and requires only molecular oxygen and no other cofactors. As a result GFP can be expressed in fluorescent form in essentially any prokaryotic or eukaryotic cell (5). GFP has been engineered to produce a vast number of variously colored mutants including blue, cyan and yellow protein derivatives, BFP, CFP and YFP (6-9). GFP and other fluorescent proteins derived from jellyfish, coral and other Cnidaria are widely used as tracers in transfection and transgenic experiments to monitor gene expression and protein localization in vivo and in in vitro. The crystal structure of GFP was determined (7) which allowed amino acid modifications to improve spectral properties and prevent multimerization (8,9). The 2008 Nobel prize in chemistry was awarded “for the discovery and development of the green fluorescent protein, GFP”.
The PROT-r-AcGFP protein originates from an Aequoria coerulescens, a close relative of A. victoria, and the protein was engineered to improve spectral properties and prevent oligomerization (10). This form of GFP, referred to as AcGFP, is 94% identical to the eGFP developed by Tsien and coworkers and is the form of GFP inserted in the Clontech/Takara pAcGFP and related expression vectors. We also supply mouse monoclonal antibodies and rabbit, chicken and goat polyclonal antibodies to this protein, MCA-3B11, MCA-1F1, RPCA-GFP, CPCA-GFP and GPCA-GFP.
Sequence taken from AY233272, download here, also Uniprot entry Q6YGZ0. This antibody was made against a recombinant construct expressed in and purified from E. coli using the pET29a (+) vector. This vector adds a few N and C-terminal amino acids which are underlined below. The N terminus contains an S-tag, highlighted in red below, which can be used to purify the protein. However we used the C-terminal His-tag (green below) to purify the protein. The sequence is identical to that found in a series of widely used expression vectors.
MKETAAAKFE RQHMDSPDLG TLVPRGSMAD IGSEFMVSKG AELFTGIVPI LIELNGDVNG 60
HKFSVSGEGE GDATYGKLTL KFICTTGKLP VPWPTLVTTL SYGVQCFSRY PDHMKQHDFF 120
KSAMPEGYIQ ERTIFFEDDG NYKSRAEVKF EGDTLVNRIE LTGTDFKEDG NILGNKMEYN 189
YNAHNVYIMT DKAKNGIKVN FKIRHNIEDG SVQLADHYQQ NTPIGDGPVL LPDNHYLSTQ 240
SALSKDPNEK RDHMIYFGFV TAAAITHGMD ELYKVDKLAAALE HHHHHH 289
Number of amino acids: 289
Molecular weight: 32414.49
Theoretical pI: 5.78
Amino acid composition:
Ala (A) 18 6.2%
Arg (R) 8 2.8%
Asn (N) 15 5.2%
Asp (D) 21 7.3%
Cys (C) 2 0.7%
Gln (Q) 8 2.8%
Glu (E) 19 6.6%
Gly (G) 25 8.7%
His (H) 16 5.5%
Ile (I) 16 5.5%
Leu (L) 21 7.3%
Lys (K) 21 7.3%
Met (M) 10 3.5%
Phe (F) 15 5.2%
Pro (P) 12 4.2%
Ser (S) 14 4.8%
Thr (T) 19 6.6%
Trp (W) 1 0.3%
Tyr (Y) 12 4.2%
Val (V) 16 5.5%
Total number of negatively charged residues (Asp + Glu): 40
Total number of positively charged residues (Arg + Lys): 29
Extinction coefficients are in units of M-1 cm-1, at 280 nm measured in water.
Ext. coefficient 23505
Abs 0.1% (=1 g/l) 0.725, assuming all pairs of Cys residues form cystines
Ext. coefficient 23380
Abs 0.1% (=1 g/l) 0.721, assuming all Cys residues are reduced
l. Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 3:223–39 (1962).
2. Shimomura, O. Structure of the chromophore of Aequorea green fluorescent protein. FEBS Lett. 104:220–2 (1979).
3. Prasher DC, et al. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111:229-33 (1992).
4. Cody CW, et al. Chemical structure of the hexapeptide chromophore of the Aequorea green-fluorescent protein. Biochem. 32:1212-8 (1993).
5. Chalfie M, et al. Green Fluorescent protein as a marker for gene expression. Science 263:802-5 (1994).
6. Heim R, Prasher DC, Tsien RY. Wavelength mutations and post-translational autoxidation of green fluorescent protein. PNAS 91:12501-04 (1994).
7. Ormo M, et al. Crystal structure of the Aequorea victoria green fluorescent protein. Science 273:1392-95 (1996).
8. Tsien RY. The green fluorescent protein. Annu. Rev. Biochem. 67:509-44 (1998).
9. Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296:913-6 (2002).
10. Gurskaya NG, et al. A colourless green fluorescent protein homologue from the non-fluorescent hydromedusa Aequorea coerulescens and its fluorescent mutants. Biochem. J. 373:403-8 (2003).
Related products
-
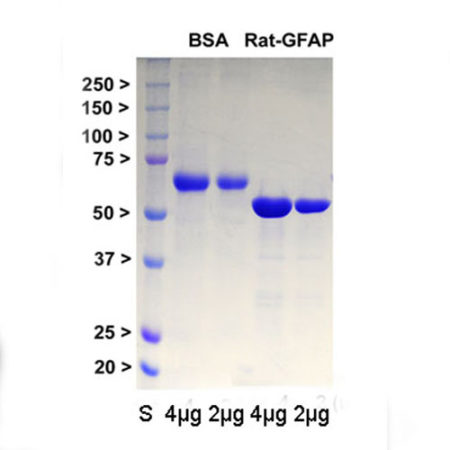
Recombinant Rat GFAP
$300.00 – $2,000.00
Cat# Prot-r-GFAP-ratSelect options This product has multiple variants. The options may be chosen on the product page -
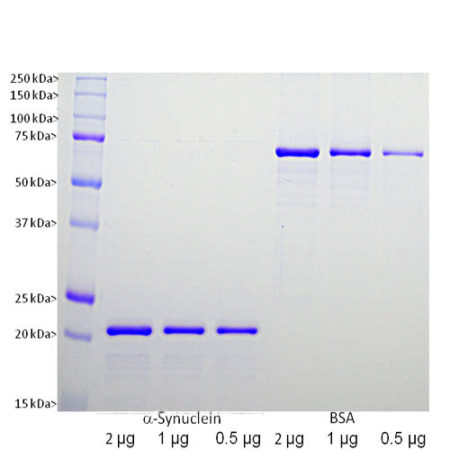
Human α-Synuclein Protein,
$300.00 – $2,000.00
Cat# Prot-r-SNCASelect options This product has multiple variants. The options may be chosen on the product page -
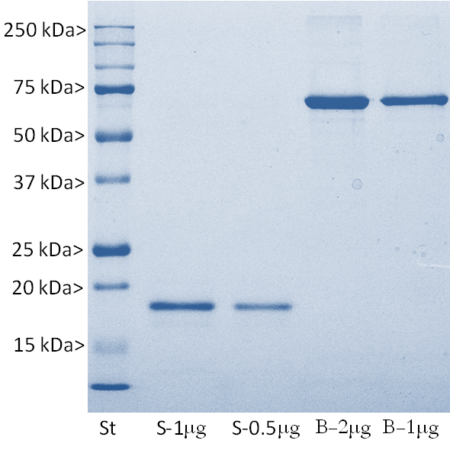
Recombinant Human S100β Protein, Cat# Prot-r-S100B
$300.00 – $2,000.00Select options This product has multiple variants. The options may be chosen on the product page -
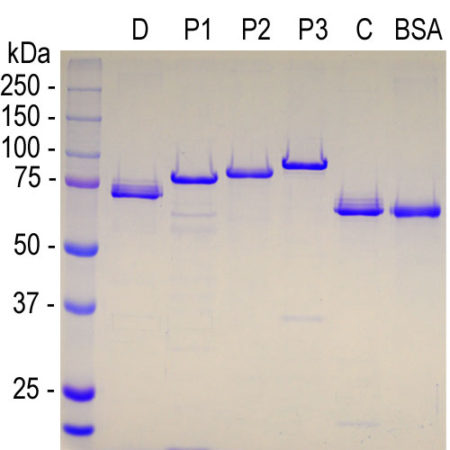
Human MAP2 Projection P2
$300.00 – $2,000.00
Cat# Prot-r-MAP2A/B-P2Select options This product has multiple variants. The options may be chosen on the product page
Contact info
EnCor Biotechnology Inc.
4949 SW 41st Boulevard, Ste 40
Gainesville
Florida 32608 USA
Phone: (352) 372 7022
Fax: (352) 372 7066
E-mail: admin@encorbio.com