EnCor Biotechnology
Mouse Monoclonal Antibody to Glyceraldehyde-3-Phosphate Dehydrogenase(GAPDH), Cat# MCA-1D4
Description
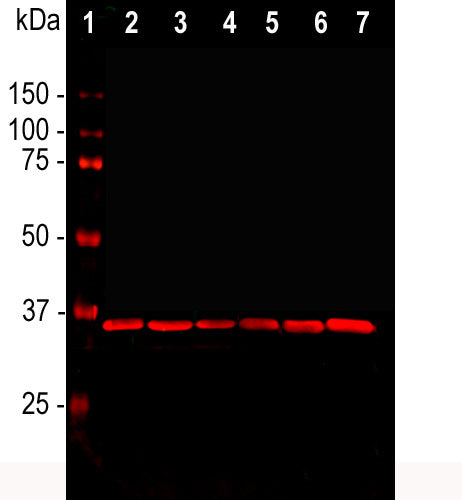
This antibody was raised against extensively purified pig GAPDH. The antibody has been widely used as a western blotting standard by many labs for many years and is known to detect GAPDH in a variety of mammalian species including human, rat and mouse, see here. We mapped the epitope for the antibody to within the peptide KYDDIKKVVKQASEGPLKGI, amino acids 254-273 of the human sequence, a peptide which is highly conserved across mammalian species. The antibody produces a single clean band on western blots of typical cell and tissue extracts. We also market a widely used rabbit polyclonal antibody to GAPDH, RPCA-GAPDH with similar properties to MCA-1D4.
- Cytoplasmic Enzyme
- Epitope Mapped Antibodies
- Immunohistochemistry Verified
- Mouse Monoclonal Antibodies
- Pathology Related Marker
Add a short description for this tabbed section
| Immunogen: | Full length protein purified from pig blood cells |
| HGNC Name: | GAPDH |
| UniProt: | P00355 |
| Molecular Weight: | 36kDa |
| Host: | Mouse |
| Isotype: | IgM heavy, κ light |
| Species Cross-Reactivity: | Human, rat, mouse, cow, pig, horse, monkey, dog, chicken |
| RRID: | AB_2107599 |
| Format: | Protein L affinity purified antibody at 1mg/mL in 50% PBS, 50% glycerol plus 5mM NaN3 |
| Applications: | WB, ICC, IHC |
| Recommended Dilutions: | WB: 1:2,000. ICC: 1:100. IHC: 1:1,000-1:2,000. |
| Storage: | Store at 4°C for short term, for longer term store at -20°C. Stable for 12 months from date of receipt. |
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is a metabolic enzyme responsible for catalyzing one step in the glycolytic pathway, the reversible oxidative phosphorylation of glyceraldehyde 3-phosphate to produce glyceraldehyde 1-3-bisphosphate. Because GAPDH protein is expressed in large amounts and is required at all times for an important “house keeping” function, levels of GAPDH mRNA are often measured and used as standards in studies of the expression of other mRNAs. Similarly specific antibodies to GAPDH are used to measure GAPDH expression as a protein standard in western blotting experiments, allowing comparison between the level of this protein and others in a cell or tissue. Apart from a role in glycolysis, GAPDH may have other roles, such as in the activation of transcription (1), and binds to a variety of other proteins, including the amyloid precursor protein, the polyglutamine tracts of Huntingtin (2,3). The protein may also have a role in the regulation of apoptosis, and interestingly migrates from the cytoplasm into the nucleus when cells become apoptotic (4). The control of this is mediated by NO mediated S-nitrosylation of GAPDH in the cytoplasm which then binds to and stabilizes the Siah1 E3 ubiquitin ligase which enters the nucleus along with nitrosylated GAPDH and initiates specific protein degradation events leading to the activation of a nuclear cell death pathway (5). Recently GAPDH was shown to be a substrate for the 5'-AMP dependent protein kinase which results in the redistribution of cellular GAPDH to cytosolic membranes and the inhibition of intracellular transport, a component of the cellular response to starvation (6).

Chromogenic immunostaining of a NBF fixed paraffin embedded human pancreas section with mouse mAb to GAPDH, MCA-1D4, dilution 1:1,000, detected with DAB (brown) using the Vector labs ABC method and goat anti-mouse IgM secondary with citrate buffer retrieval. Hematoxylin (blue) was used as the counterstain. The GAPDH antibody strongly labels pancreatic exocrine glandular cells. This antibody performs well in testing with both 4% PFA and standard NBF fixed tissues. Mouse select image for larger view.
This antibody has been widely used for many years as it has been marketed through several OEM partners of EnCor. Since we have sold a large amount of this antibody directly to researchers, several publications cite out company as the source of this antibody, see CiteAb citations for EnCor MCA-1D4.
Some idea of how widely used is this antibody can be obtained from a Google Scholar search for peer-reviewed publications in which it was used. Here is a list of other peer reviewed publications which make use of this antibody as supplied by EnCor.
1. Fortun J, et al. Emerging Role for Autophagy in the Removal of Aggresomes in Schwann Cells. J. Neurosci. 23:10672-80 (2003).
2. Ellis RC, et al. Cathepsin B mRNA and protein expression following contusion spinal cord injury in rats. J. Neurochem. 88:689-97 (2004).
3. Fortun J, et al. Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J. Neurochem. 92:1531-41 (2005).
4. Fortun J, Li J, Go J, Fenstermaker A, Fletcher BS & Notterpek L. Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J. Neurochem. 93:766-8 (2005).
5. Iskandar M, et al. Copper chaperone for Cu/Zn superoxide dismutase is a sensitive biomarker of mild copper deficiency induced by moderately high intakes of zinc. Nutr. J. 4:35 (2005).
6. Fortun J, et al. Alterations in degradative pathways and protein aggregation in a neuropathy model based on PMP22 overexpression. Neurobiol. Dis. 22:153-164 (2006).
7. Amici SA, et al. Peripheral Myelin Protein 22 Is in Complex with {alpha}6beta4 Integrin, and Its Absence Alters the Schwann Cell Basal Lamina. J. Neurosci. 26:1179-89 (2006).
8. Amici SA, Dunn WA, Notterpek, L. Developmental abnormalities in the nerves of peripheral myelin protein 22-deficient mice. J. Neurosci. Res. 85:238-49 (2006).
9. Felitsyn N, Stacpoole, PW, Nottepek L. Dichloroacetate causes reversible demyelination in vitro: potential mechanism for its neuropathic effect. J. Neurochem. 100:429-36 (2007).
10. Rangaraju S, et al. Pharmacological induction of the heat shock response improves myelination in a neuropathic model. Neurobiol Dis. 32:105-15 2008
11. Felitsyn N, et al. The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J Neurochem. 106:2068-79 2008
12. Lau P, et al. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 28:11720-30 2008.
13. Verrier JD, et al. Peripheral myelin protein 22 is regulated post-transcriptionally by miRNA-29a. Glia 57:1265-79 2009.
14. Rangaraju S, et al. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 8:178-91 2009.
15. Madorsky I, et al. Intermittent fasting alleviates the neuropathic phenotype in a mouse model of Charcot-Marie-Tooth disease. Neurobiol Dis. 34:146-54 2009.
16. Opalach K, et al. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 13:65-74 2010.
17. Verrier JD, et al. Reduction of Dicer impairs Schwann cell differentiation and myelination. J. Neurosci. Res. 88:2558-68 2010.
18. Zeier Z, et al. Gene Expression in the Hippocampus: Regionally Specific Effects of Aging and Caloric Restriction. Mech. Ageing Dev. 132:8-19 2011
19. Verrier JD, et al. Bicistronic lentiviruses containing a viral 2A cleavage sequence reliably co-express two proteins and restore vision to an animal model of LCA1. PLoS One. 6:e20553 2011.
20. Lee WH, et al. Influence of viral vector-mediated delivery of superoxide dismutase and catalase to the hippocampus on spatial learning and memory during aging. Antioxid Redox Signal. 16:339-50 2012.
21. Kumar A, et al. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging 33 828,e1-17 (2011).
1. Morgenegg G, et al. Glyceraldehyde-3-phosphate dehydrogenase is a nonhistone protein and a possible activator of transcription in neurons. J. Neurochem. 47:54-62 (1986).
2. Schulze H, et al. Rat brain glyceraldehyde-3-phosphate dehydrogenase interacts with the recombinant cytoplasmic domain of Alzheimer’s beta-amyloid precursor protein. J Neurochem. 60:1915-22 (1993).
3. Burke JR, et al. Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nature Med. 2:347-50 (1996).
4. Dastoor Z, Dreyer J-L. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J. Cell Sci. 114:1643-53 (2001).
5. Hara MR, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 7:665-74 (2005).
6. Yang J-S, et al. GAPDH inhibits intracellular pathways during starvation for cellular energy homeostasis. Nature 561:263-67 (2018).
Since we have sold large amounts of this antibody directly to researchers, several publications cite our company as the source of this antibody, see CiteAb citations for EnCor MCA-1D4.
Some idea of how widely used is this antibody when sold through our numerous OEM partners can be obtained from a Google Scholar search for "GAPDH AND 1D4 AND Antibody" or by selecting here.
Add a short description for this tabbed section