We release two new protein constructs, PROT-r-NF-L-Rct and PROT-r-NF-L-Stan. These constructs are designed to be used as protein standards for current neurofilament NF-L antibody based assays, such as the Uman/Quanterix NF-LIGHT™ and Quanterix Simoa™ assays. The two antibodies used in these assays both bind to the center of the “Coil II” region of α-helical “Rod” region of the NF-L molecule. The PROT-r-NF-L-Rct contains contains the entire Coil II region. The PROT-r-NF-L-Stan constructs contains the epitopes for both antibodies with little flanking sequence and is engineered to include 2 tryptophan residues. The result is a convenient low molecular weight protein standard which due to the high tryptophan content can be quantified spectrophotometrically more accurately than native or recombinant NF-L. Details of the mapping of the relevant NF-L assay antibodies and our generation of a novel panel of similar products are described in a recent BioRχiv publication, 10.1101/2022.08.27.504533v1.
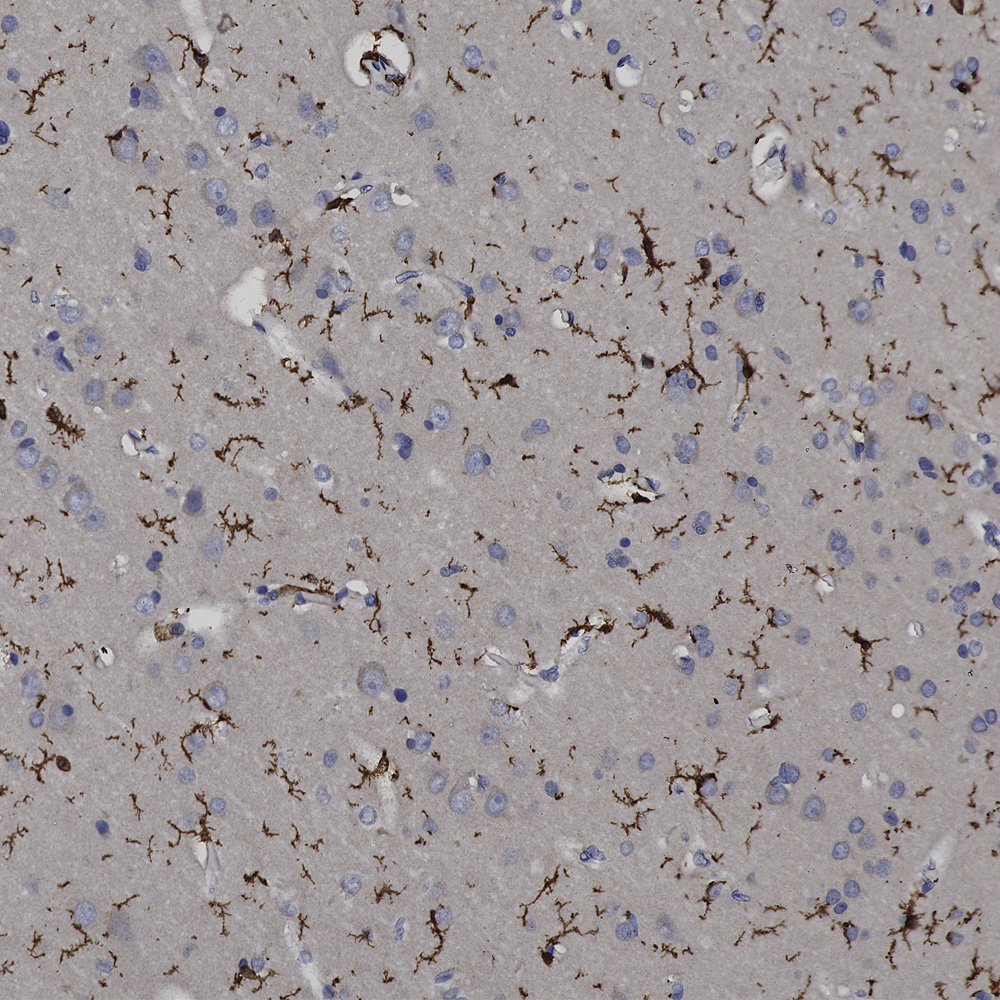
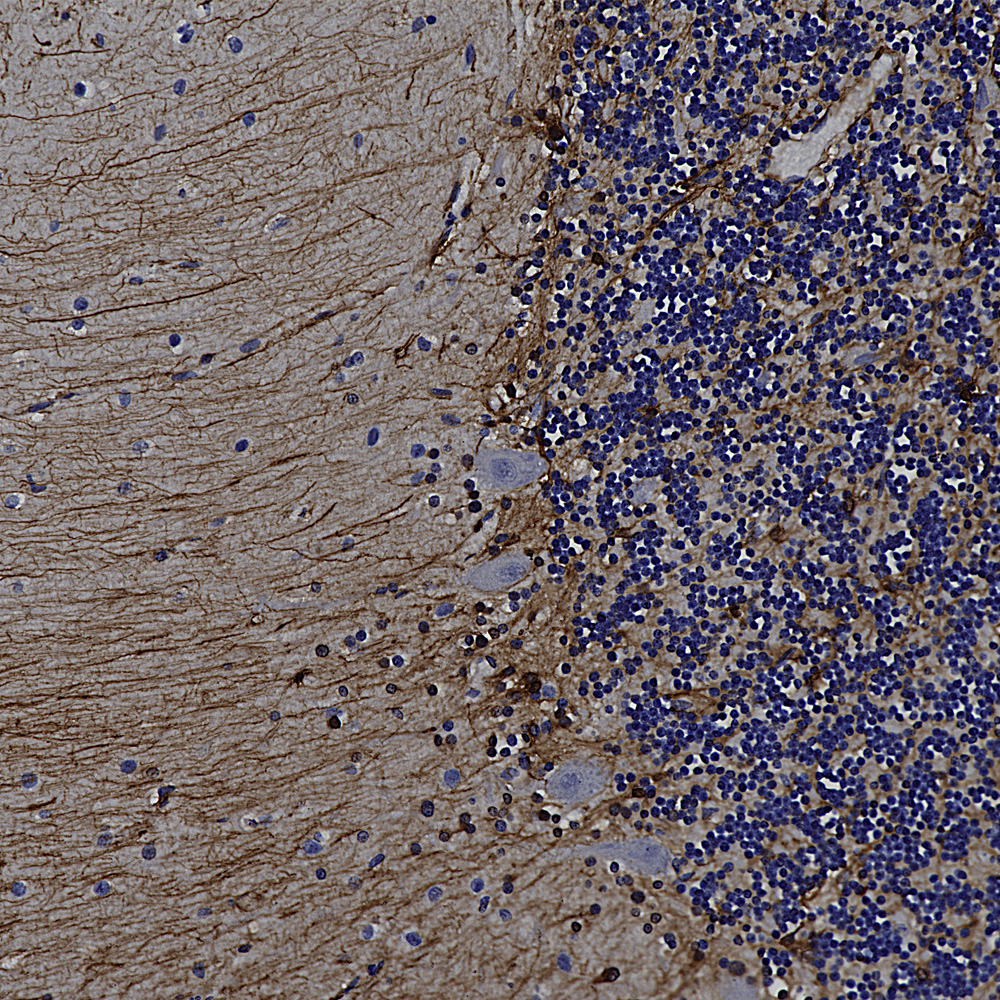
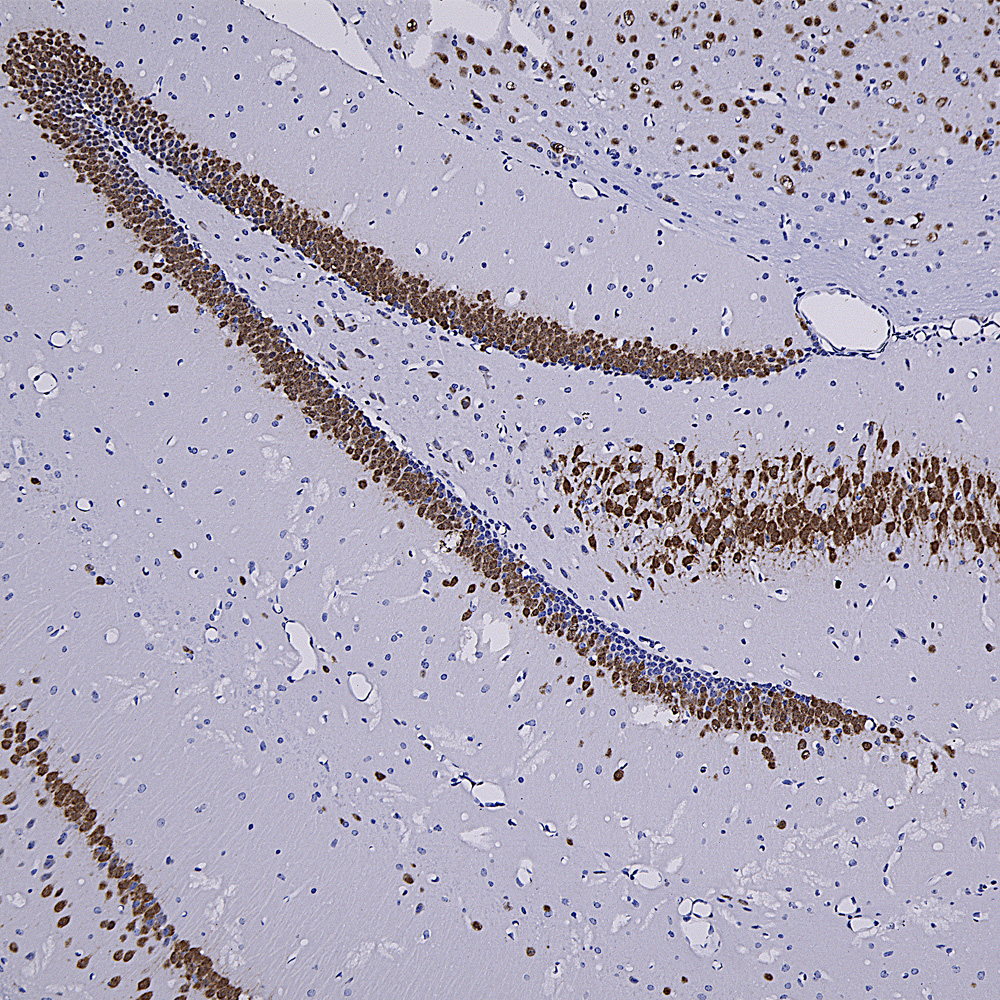
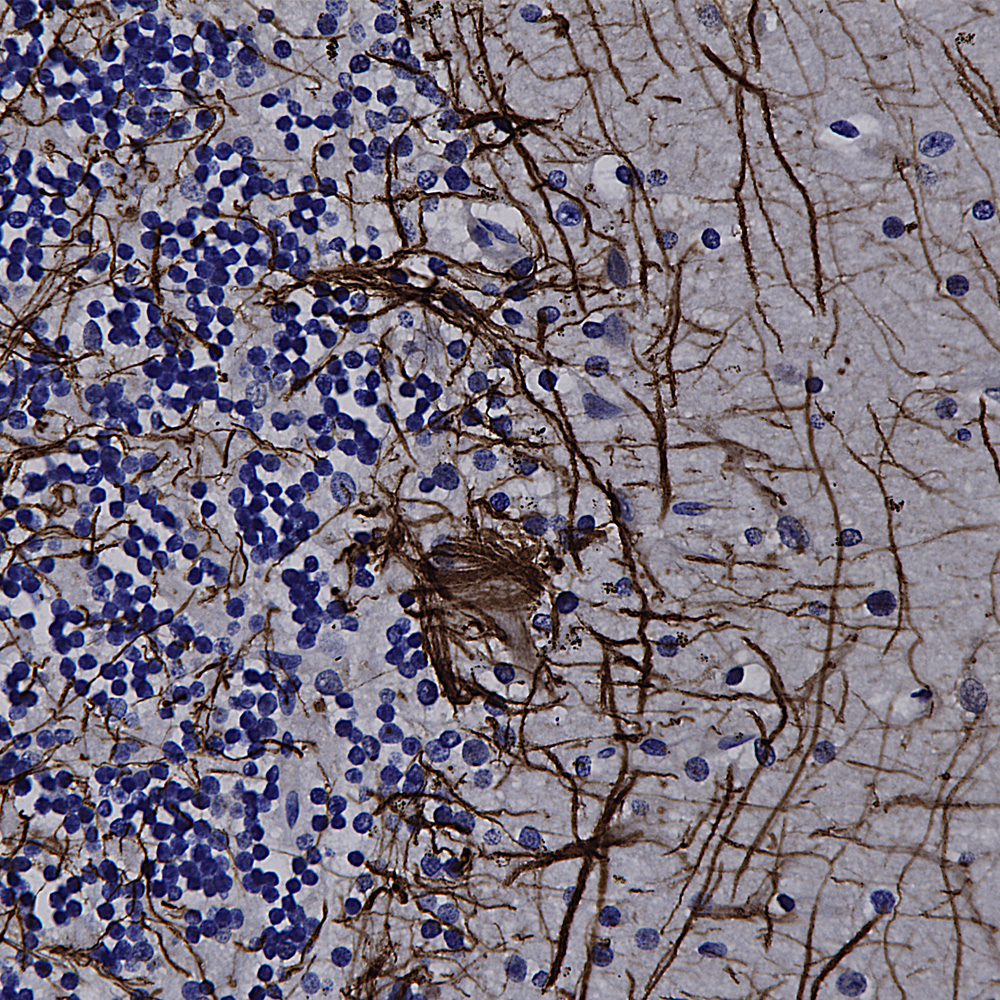
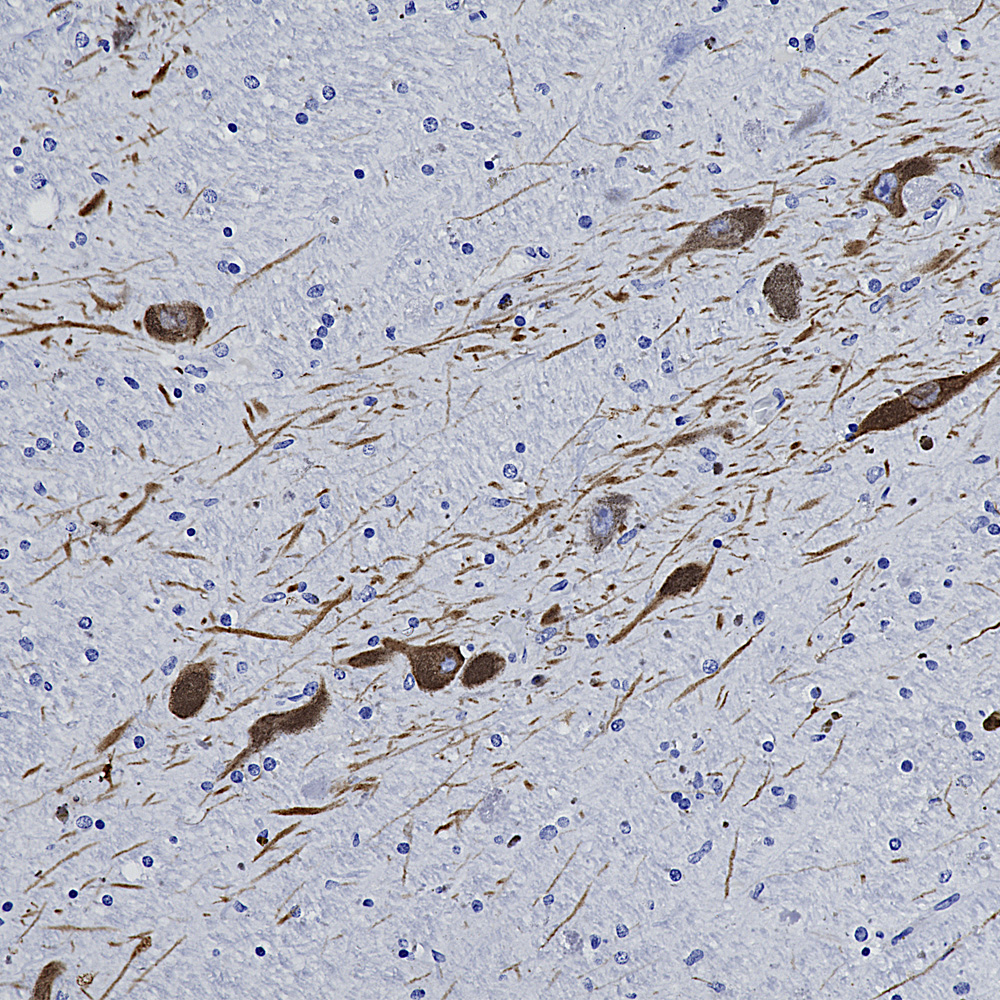
We hired an excellent immunohistochemist to the great dismay of the University of Florida so we will be validating all our antibodies on formalin fixed paraffin embedded sections, in other words for immunohistochemistry (IHC). The harsh fixation and processing typically used with IHC heavily modifies immunogens and it is therefore expected to cause some otherwise useful antibodies to not work well. However certain other antibodies may recognize epitopes which are not damaged by IHC and so should work just fine. So we added to the “Additional Info” tag of the relevant product web pages images and details of positive IHC results. We now present data on many of our chicken antibodies; Specifically MAP-τ CPCA-Tau, neurofilament NF-H CPCA-NF-H, neurofilament NF-L CPCA-NF-L, tyrosine hydroxylase CPCA-TH, FOX3/NeuN CPCA-FOX3, FOX2 CPCA-FOX2, mCherry CPCA-mCherry, GFP CPCA-GFP, GFAP CPCA-GFAP, IBA1 CPCA-IBA1, MAP2A/B CPCA-MAP2, calbindin CPCA-Calb, myelin basic protein CPCA-MBP, CNPase CPCA-CNP, secretogogin CPCA-SCGN, α-synuclein CPCA-SNCA and visinin-like protein 1 CPCA-VLP1. We have also validated several goat antibodies for formalin fixed paraffin embedded IHC including our antibody to CNPase GPCA-CNP, FOX3/NeuN GPCA-FOX3, GFAP GPCA-GFAP, GFP GPCA-gfP,
MAP2 GPCA-MAP2, MBP GPCA-MBP, mCherry GPCA-mCherry, NF-H GPCA-NF-H and tyrosine hydroxylase GPCA-TH. We have also validated some of our mouse monoclonal antibodies on paraffin embedded IHC, such as DegenoTag™ NF-L antibody MCA-1B11, antibody to mCherry MCA-1C51,
Over the next few weeks we will validate all of our other chicken, mouse, rabbit and goat antibodies for IHC on human and rodent tissues, significantly enhancing their utility. We have already validated certain of our most widely used monoclonal antibodies such as MCA-1B7 which binds to FOX3/NeuN, an excellent marker of neurons, our rabbit polyclonal antibody to IBA1, RPCA-IBA1 an excellent marker of microglia and our monoclonal antibody to ubiquitin MCA-UBI-1, an excellent marker of Alzheimer neurofibrillary tangles and other pathological inclusions.
We usually go to the normally annual Society for Neuroscience Meeting, but missed 2020 and 2021 due to the pandemic. This year the meeting was in San Diego, November 11-16, and it was fun to see various people for the first time in three years. We were at booth 401 and handed out ~1,000 of our well known poster images for free, and they had all gone by the middle of the second day. We took along a few of our slides of brain sections and HeLa cell stained with various of antibodies giving 5 distinctly different fluorescence signals at 405nm, 488nm, 541nm, 645nm and 750nm. So on the brain sections it is possible to visualize on one section some combination of nuclei, neuronal cell bodies, neuronal dendrites, myelin sheaths, axons, astrocytes and microglia. In the HeLa cells you could see some combination of nucleus, mitochondria, microfilaments, intermediate filaments, microtubules, plasma membrane, nucleoli and nuclear lamina. It turned out that the various microscope companies loved these, as they had apparently all been looking for such samples to demonstrate how their various 2 photon, confocal, fluorescence etc. microscopes worked. So we are starting to market these as a new line of products. For no particular reason Dr. Shaw also brought along some Ramon-Y-Cajal images which he had burned into wood using a laser and tastefully framed. These turned out to be very popular also, and we sold all of them. So there is yet another potential line of products, watch this space!